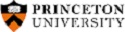Day 1 :
Keynote Forum
Paul O’Connor
ANTECY – Fruitful Innovations, Netherlands
Keynote: CREATING THE RIGHT (GREEN) CHEMISTRY CO2, Water and Biomaterials
Time : 10:00-10:40

Biography:
Paul O’ Connor graduated at the Eindhoven University of Technology in Chemical Engineering in 1977. He has been active in heavy oil conversion processes at Shell and at Akzo Nobel in development of refining catalysts. In 2006 he formed BIOeCON, focused on the economical conversion of biomass. BIOeCON has developed several breakthrough concepts, most recently a process towards selective biomass fractionation producing high value materials. In 2010 Paul formed ANTECY aiming to convert renewable energy directly into high-density liquids. ANTECY has developed technology for the capturing of CO2 based on a low cost and environmentally friendly non-amine sorbents.
Abstract:
In the billion year history of our earth a most amazing and unique process has occured of the massive conversion of the plentitude of the present CO2 in the atmosphere with water into biomass or biomaterials under the influence of solar energy, a process we call: photosynthesis.
Because of this processs the CO2 in the earth’s atmosphere dropped from 20% to ± 250 to 300ppm. Part of these biomaterials over billions of years has been degraded (or “fossilized”) and converted into coal, oil and gas, or what we call today fossil fuels.
The accelerated use of these fossil resources over the last 200 years has lead to a sharp increase in CO2, now already at 400 ppm and also the increase of Methane (CH4) in the atmosphere, triggering global warming.
It is our responsibility to invent, develop and apply the right green chemistry making use of the available natural resources such as CO2, water and biomaterials in a way which does not harm the eco-systems of our earth, meaning in a circular and sustainable way,
Examples will be given of innovations in this exciting field over the last 15 years, leading to new technologies, opening up the possibilities for:
- Advanced materials and chemicals from biomass and biomass waste
- Fuels and chemicals from CO2 and water from the open Air, making use of clean renewable energy (Solar, Wind, Hydropower etc).
Keynote Forum
Magrini Kimberly,
National Renewable Energy Laboratory, USA
Keynote: Upgrading Biomass Pyrolysis Vapors to Hydrocarbon Fuels and Chemicals

Biography:
Dr. Magrini is a Principal Scientist in the National Bioenergy Center and currently manages NREL’s Thermochemical Sciences and Engineering Group, which focuses on the development of catalytic approaches to biofuels production from syngas and pyrolysis products from biomass. She has 29 years of research and management experience in academic, industrial and national laboratory environments and has over 100 peers- reviewed publications, 2 patent applications, 1 patent and 125 presentations at national and international meetings.
Abstract:
NREL’s thermochemical biomass conversion research is focused on ex-situ catalytic fast pyrolysis also called vapor phase upgrading (VPU) as a potentially efficient and economical route to pyrolysis-based fuel precursors, fuels and chemicals. In this approach, biomass vapors are generated via fast pyrolysis (FP) and destabilizing vapor components (char, inorganics, tar aerosols) are removed by hot gas filtration with the conditioned vapors more amenable to catalytic upgrading via emerging and industrially available zeolites. We use a Davison circulating riser (DCR), a petroleum industry standard, for vapor phase upgrading while a close coupled pyrolyzer system produces consistent pyrolysis vapors as feed to the DCR. Concurrent upgrading catalyst development is focused on identifying and evaluating modifications to ZSM5-based catalysts that increase carbon content of the condensed product while also reducing catalyst coking and increasing deoxygenation activity. Subsequent catalyst screening for vapor upgrading showed marked differences in product composition with catalyst type while similar liquid product was obtained with both mixed hardwood and clean pine feedstocks using the same catalyst and process conditions. Ash, aerosols and char removal were additionally quantified for selected experiments. The work presented here will show 1) the impact on product composition from pure vapor upgrading with a suite of catalysts comprising unmodified, and P- and metal-modified zeolites, 2) comprehensive physical and chemical product composition, 3) product fuels from hydrotreating, and 4) chemicals from aqueous phase organics. These results will be discussed and compared with other work conducted in riser systems to produce biomass derived hydrocarbon fuels.
Keynote Forum
Anet Režek Jambrak
University of Zagreb, Croatia
Keynote: "Greener" food processing in light of sustainability
Time : 10:40-11:20

Biography:
ANET REŽEK JAMBRAK, Associate professor is working at the Faculty of Food Technology and Biotechnology of the University of Zagreb, Croatia. She is working in the area of nonthermal and advanced thermal processing techniques, food chemistry, food physics, and process engineering. She also has strong international collaboration with renowned scientists. In the period from 2007. Anet Režek Jambrak has published over 80 significant scientific papers, published in top scientific journals with high impact factors (citation more than 1300, h-index 20). She is the winner of the 2016. Young Scientist Award from the International Union of Food Science and Technology.
Abstract:
Non-thermal and innovative processing technologies are attracting great attention nowadays. The advantages of those “green” technologies lies in faster, better, cheaper, sustainable and optimised process for preservation of foods, modification of food components or to design “novel food”.
Application of thermal techniques is used for decades and non-thermal techniques are being “considered” in terms of food preservation. Non-thermal processing techniques include: electrotechnologies, UV light, cold pressure (high pressure processing), hydrodynamic cavitation, ionising radiation, ozonation, oscillating magnetic fields, pulsed light, supercritical fluid processing, biopreservation, electrohydrodynamic processing and electron beam processing.
Sustainability of non-thermal processing is now "hot" topic. Valorisation of agri-food wastes by non-thermal technologies is great research area nowadays. There is large discard of food by-products in food industry that can be used as energy or raw-material for other purposes. In order to think “green”, eco (economic, ecologic and environmental) we must think about having non-thermal processing in the way of less processing time, less energy consumption, less CO2 production and energy efficient processing (sustainability). Food scientists need to think to connect all processing variables and to have "green" strategy. There are methods of life cycle assessment (LCA); Quality function deployment etc. that can combine parameters and give results about improvement of processing, consumer’s preferences and impact on the environment. The use of "green" solvents is one example in sustainable extraction.
Non-thermal and innovative food processing can and must be optimised, and results should be transformed from lab scale to large scale (industry).
- Industrial Applications of Green Chemistry | Environmental Chemistry and Pollution Control | Green Chemistry and Technology | Biomass and its Conversion | Life Cycle Assessment and Environmental Sustainability

Chair
Paul O Connor
ANTECY, Netherlands
Co-Chair
Rudina Bleta
University of Artois, France
Session Introduction
Paul O’Connor
ANTECY – Fruitful Innovations, Netherlands
Title: CREATING THE RIGHT (GREEN) CHEMISTRY CO2, Water and Biomaterials

Biography:
Paul O’ Connor graduated at the Eindhoven University of Technology in Chemical Engineering in 1977. He has been active in heavy oil conversion processes at Shell and at Akzo Nobel in development of refining catalysts. In 2006 he formed BIOeCON, focused on the economical conversion of biomass. BIOeCON has developed several breakthrough concepts, most recently a process towards selective biomass fractionation producing high value materials. In 2010 Paul formed ANTECY aiming to convert renewable energy directly into high-density liquids. ANTECY has developed technology for the capturing of CO2 based on a low cost and environmentally friendly non-amine sorbents.
Abstract:
In the billion year history of our earth a most amazing and unique process has occured of the massive conversion of the plentitude of the present CO2 in the atmosphere with water into biomass or biomaterials under the influence of solar energy, a process we call: photosynthesis.
Because of this processs the CO2 in the earth’s atmosphere dropped from 20% to ± 250 to 300ppm. Part of these biomaterials over billions of years has been degraded (or “fossilized”) and converted into coal, oil and gas, or what we call today fossil fuels.
The accelerated use of these fossil resources over the last 200 years has lead to a sharp increase in CO2, now already at 400 ppm and also the increase of Methane (CH4) in the atmosphere, triggering global warming.
It is our responsibility to invent, develop and apply the right green chemistry making use of the available natural resources such as CO2, water and biomaterials in a way which does not harm the eco-systems of our earth, meaning in a circular and sustainable way,
Examples will be given of innovations in this exciting field over the last 15 years, leading to new technologies, opening up the possibilities for:
- Advanced materials and chemicals from biomass and biomass waste
- Fuels and chemicals from CO2 and water from the open Air, making use of clean renewable energy (Solar, Wind, Hydropower etc).
Magrini Kimberly
National Renewable Energy Laboratory, USA
Title: Upgrading Biomass Pyrolysis Vapors to Hydrocarbon Fuels and Chemicals

Biography:
Dr. Kimberly Magrini is a Principal Scientist in the National Bioenergy Center and currently manages NREL’s Thermochemical Sciences and Engineering Group, which focuses on the development of catalytic approaches to biofuels production from syngas and pyrolysis products from biomass. She has 29 years of research and management experience in academic, industrial and national laboratory environments and has over 100 peers- reviewed publications, 2 patent applications, 1 patent and 125 presentations at national and international meetings.
Abstract:
NREL’s thermochemical biomass conversion research is focused on ex-situ catalytic fast pyrolysis also called vapor phase upgrading (VPU) as a potentially efficient and economical route to pyrolysis-based fuel precursors, fuels and chemicals. In this approach, biomass vapors are generated via fast pyrolysis (FP) and destabilizing vapor components (char, inorganics, tar aerosols) are removed by hot gas filtration with the conditioned vapors more amenable to catalytic upgrading via emerging and industrially available zeolites. We use a Davison circulating riser (DCR), a petroleum industry standard, for vapor phase upgrading while a close coupled pyrolyzer system produces consistent pyrolysis vapors as feed to the DCR. Concurrent upgrading catalyst development is focused on identifying and evaluating modifications to ZSM5-based catalysts that increase carbon content of the condensed product while also reducing catalyst coking and increasing deoxygenation activity. Subsequent catalyst screening for vapor upgrading showed marked differences in product composition with catalyst type while similar liquid product was obtained with both mixed hardwood and clean pine feedstocks using the same catalyst and process conditions. Ash, aerosols and char removal were additionally quantified for selected experiments. The work presented here will show 1) the impact on product composition from pure vapor upgrading with a suite of catalysts comprising unmodified, and P- and metal-modified zeolites, 2) comprehensive physical and chemical product composition, 3) product fuels from hydrotreating, and 4) chemicals from aqueous phase organics. These results will be discussed and compared with other work conducted in riser systems to produce biomass derived hydrocarbon fuels.
Dimitris S. Argyropoulos
North Carolina State University, USA
Title: Toward Carbon Fibers from Single Component Kraft Lignin Systems; An Application of Green Chemistry with Forest Biomaterials

Biography:
Dr. Dimitris Argyropoulos, Professor of Chemistry at North Carolina State University, is internationally recognized for his leading contributions to Green Chemistry using wood biopolymers. His work focuses at promoting our understanding of the structure and reactivity of lignin and the development novel NMR and material science techniques for the structural elucidation and the upgrading of these biopolymers representing otherwise unsolved, intractable problems in lignin based material’s chemistry. The efforts of his research group have been disseminated in excess of 200 scientific papers, numerous scientific conferences and invited presentations.
Abstract:
Carbon fibers represent a class of materials with enormous potential for many material and other engineering applications for our society. There are projections that by 2020 the actual demand for carbon fibers will be such that the traditional poly-acrylonotrile precursors used today will not be enough to address the projected demand. Consequently, it is imperative that new precursors based on the foundations of Green Chemistry need be developed. In this respect technical lignins present us with formidable challenges but also with enormous opportunities and they are to be explored in detail during this presentation. In our earlier effort we have embarked in describing and discussing the importance of propargylation chemistry on lignin so as to synthesize lignin macromonomers for thermal polymerization via Claisen rearrangement 1, 2. We have also discussed that the molecular weight and glass transition temperatures of the thermally polymerized lignin improves significantly relative to the starting material. The intricate polymer structure created within lignin as a result of the benzopyran double bond thermal polymerization chemistry is offering a regular covalently linked framework from which, after carbonization, a regular carbon fiber material could. As such, thermally polymerized propargylated softwood lignin emerges as a prospective material for the synthesis of bio-based Carbon Fiber precursor. Various reactivity considerations that are to be discussed in the presentation 3 were addressed by a series of experiments where initially Acetone Soluble Kraft Lignin (ASKL) was propargylated, thus occupying all readily accessible and highly reactive phenolic–OHs, followed by methylation of the remaining phenolic OH’s to limit phenoxy radical induced thermal polymerization. All the polymerization reactions were conducted by heating the samples at 180 °C for three hours and the corresponding molecular weights and distributions were determined.
As anticipated, the installation of the propargyl groups in more reactive positions, more readily prone to Claisen rearrangement and thermal polymerization events, offered much better developed molecular weights able to offer Carbon Fibers.
Claudia Crestini
University of Rome Tor Vergata, Italy
Title: Tannins: a new approach to characterization, chemical modification and processing towards innovative products and nano materials

Biography:
Claudia Crestini is associate professor at the University of Rome Tor Vergata. Director of the Laboratory of Polyphenols Chemistry & Materials Science, her work is internationnally recognized as a leading contribution to Green Chemistry using natural polyphenols. It is focused on the development of new methods of structural analysis of polyphenolic polymers, development of new materials and products by chemical/biotechnological modification and development of innovative stimuli responsive nanomaterials from natural polyphenols. She has published more than 140 publications on international refereed journals and over 150 contributions to international conferences and invited presentations. H-index 41, citations > 5000 (source google scholar).
Abstract:
Tannins are natural polyphenols found in most higher plants around the globe. They play a significant role in defending the plant against insects, infections, fungi or bacteria; this role stems from their capability to form complexes with proteins, polysaccharides and metals, and hence provide protection to the vulnerable parts of the plants against invasive microbial extracellular enzymes. However, their exploitation as renewable high added value products is to date not extensive despite their interesting intrinsic properties, including high biocompatibility and biodegradability. The fundamental positive health effects of tannins, which are connected to their high antioxidant activity and their role as radical scavengers, allow for protection from diseases associate with the presence of free radicals in the body, such as cancer, arthritis, and degenerative eye and neurological disorders, and display significant potential for biofilm control undoubtedly revealing intriguing potential for their application in biomedical fields that is yet to be explored. In this frame our research group, aiming at designing a rational process for tannins valorization developed an innovative 31P NMR analytical technique for fast and reliable quantification of all the different phenolic groups present in complex tannns matrices and applied it to the selective functionalization of tannins of different origins and structures in order to tune biological and chemico physical properties such as hydrophobicity and chelation. Furthermore, the high tendency to supramolecular interactions was succesfully exploited for the design and development of nanostructures for synergistic controlled drug delivery by ultrasonication.
Thorsten Brandau
Brace GmbH, Germany
Title: Powering Green Chemistry with Microspheres and Microcapsules
Time : 11:40- 12:10

Biography:
Thorsten Brandau completed his PhD at Goethe University. He is president at BRACE GmbH, Germany.
Abstract:
In Green Chemistry processes, there is a need for administering actives in well defined forms as well as using processes and materials in a recoverable and sustainable manner. For these needs, it is mandatory to discuss shape, size and form of dosage, carriers and recovery methods.
Microspheres and Microcapsules manufactured with the BRACE Microsphere processes offer the unique possibility to combine encapsulation with low energy production process as well as recovering of carriers with resource saving means.
The monomodal size and extremely tight size distribution of the particles produced with such processes, allow the precise dosage or the handling of catalysts in a most advantageous manner. Particles produced with the patented BRACE-microsphere processes can be used for producing catalysts and catalyst carriers in a size range from about 50 micrometers up to 8 mm, or the encapsulation of an extremely wide range of materials for release on definable triggers – such as mechanical force, temperature, pH, solubility, and many others. The processes can be used to reform materials from sub-zero to 1500°C, while most materials can be easily processed at room temperature to form perfectly round spheres. Such spheres show extremely well definable release properties and can be tailored to almost any application. Applications so far realized range from catalysts and catalyst carriers – easily recoverable and reusable –, over bioreactors, cell encapsulation for biochemical processes, agricultural applications – such as reducing the pesticide and fertilizer needs –, to energy processing for sustainable construction materials, recovering oil and gas, solar cells, energy storage and many more applications.
In the field of alternative fuel production or the thermal conversion of biomass, there are several applications already that make use of those properties. As the scalability of the processes is easy, straight forward and unlimited, also large and very large scale productions can easily be covered, at both, a low energy and resource use that scales less than the production output.
Rudina BLETA
University of ARTOIS, France
Title: Cyclodextrins as Versatile Tools for the Preparation of UV- and Visible-light Responsive Mesoporous Photocatalysts
Time : 12:10-12:40

Biography:
Rudina Bleta has completed her PhD from Nancy University and postdoctoral studies from University Paul Sabatier at the CIRIMAT-Carnot Institute in Toulouse. In 2012, she joined the Professor Monflier’s team at the UCCS-Artois as a lecturer. Her research expertise consists in developing new synthesis approaches, especially from soft chemistry routes, to design novel nanostructured porous materials, with a specific focus on the development of heterogeneous catalysts for environmental and sustainable energy applications.
Abstract:
The development of sustainable chemical processes is becoming a major feature of research for the protection of human health and the environment. In this context, the heterogeneous photocatalysis, using semiconductor-liquid interfaces as catalytic sites for solar light-stimulated redox reactions, has emerged as a promising technology for environmental clean-up applications. Among the various metal oxide semiconductors, titanium dioxide (TiO2) has become one of the most important photocatalysts because of its chemical stability and unique ability in catalyzing water splitting, air purification and water decontamination. For effective solar energy utilization, modification of TiO2 surface with noble metal nanoparticles provides an alternative approach for extending the absorption wavelength from the ultraviolet (UV) to the visible region. In this context, Au/TiO2 composites have attracted much interest as efficient plasmonic photocatalysts owing to the ability of Au nanoparticles to absorb light in the visible region and TiO2 to efficiently separate the photogenerated electrons and holes at the metal-semiconductor interface.
In this work, we describe a simple colloidal self-assembly approach towards highly active UV- and visible-light photocatalysts that takes advantage of the ability of cyclodextrins to direct the self-assembly of TiO2 colloids in a porous network over which Au nanoparticles can be uniformly dispersed. The performance of these nanocomposites is evaluated in the visible light photocatalytic degradation of the phenoxyacetic acid (PAA), a widely utilized herbicide, frequently detected in natural water. The CD-driven approach is simple and provides a versatile route towards a broad range of nanostructured composites with promising properties for environmental clean-up applications.
Elze C. van Hamelen
The Natural Step, Germany
Title: Systems approach to identify sustainable chemical innovations that encompass life-cycle impacts

Biography:
Elze is an Advisor at The Natural Step Germany, and Associate Partner at the Sustainable Growth Associates network. Elze holds an MBA in Sustainable Management and an MA degree in Organisational Sociology, combining different perspectives within an organisation, such as policy, strategy, change, culture, cooperation, behaviour and communication. Elze is passionate about the strategic implementation of sustainability using The Natural Step framework, and she’s convinced that innovations in chemistry and materials management are key to accelerating the transition to sustainability.
Abstract:
Too often we cherry-pick sustainability topics: there is a focus on green chemistry, climate change, on circular economy, or types of poverty. Rarely do we look at the many faceted challenges of sustainability as deeply interconnected, The root causes of these challenges lie in the fundamental unsustainability of the way we organize our businesses and societies.
Based on the laws of thermodynamics and sociological research on the healthy functioning of socio-ecological systems, the Framework for Strategic Sustainable Development (FSSD) identifies four root causes of unsustainability. Together, they constitute the boundary conditions or design principles for humanities to sustain within the limits of planet earth. In this way, the conditions constitute a science-based definition of success for sustainable design, selection and management of chemicals, materials and products.
The Strategic Life Cycle Assessment (SLCA) applies the sustainability principles in each phase of the product life cycle – creating a ‘heat map’, merging perspectives on chemicals, circular economy and SDGs, and providing direction for effective sustainability innovation. By applying these sustainability principles rigorously and strategically, the systems perspective that underlies it assures holistic and integrated solutions, avoiding solutions that only combat symptoms, or solutions that cause complications in other domains.
The Natural Step has been helping organisations apply these principles since the early 1990’s. Here we focus on application and insights around circular / sustainable product innovation.
Chungsik Yoon
Seoul National University, South Korea
Title: Characteristics of Chemicals and Trade Secrets Used in the Semiconductor Manufacturing Industry
Time : 14:10-14:40

Biography:
Chungsik Yoon has completed his PhD from Seoul National Universty in 1999 and got Certified Industrial Hygienist (CIH) from the American Board of Industrial Hygiene in 2001. He is the professor of School of Public Health, Seoul National Universty and the associate president of Korean Industrial Hygiene Association. He has published more than 150 papers in reputed journals and has been serving as an editorial board member of the Safet and Health at Work journal.
Abstract:
The semiconductor industry is considered the most high technology based industry and is characterized by complicated processes as well as massive chemical use. This study aimed to know the overall status of the chemical use, trade secrects and to evaluate the high risk CMR(carcinogens, mutagens and reproductive toxic) chemicals used in semiconductor manufacturing industries. On behalf of OSHRI (Occupational Safety and Health Research Institute) Project in Korea, we collected data about Serial no. chemical product name, chemical constituents (chemical name, CAS No.) and trade secrets, contents of each product, the physical status of chemical, amount of annual use, manufacturer and vendor of the chemical products, chemical use process from twelve workplaces. On average, 210±124 chemical products were used in semiconductor industry and 33±16% of proucts contained at least one trade sectrets. Numerous CMR substances were found including sulfuric acid, chromic acid, Ethylene oxide, hydroquinone and 2-Ethoxyethanol. More than 60% of chemical substane used in semiconductor industy has no NEFA index on health, safety and reactivity. Chemical product supplier was diverse. Average number of chemical product manufacturer was 62±21 per semiconductor plant and the number of chemical products supplied by each manufacturer was from 1 to 41 product.
High propotion of trade secrets, numerous numbers of CMR, high percentage of no health and safety information on the chemical proucts waits on proactive challenge and study for green chemistry and sustainabilty in semiconductor industry.
Yaxin Su
Donghua University, China
Title: Effect of Al3+/clay Ratio on C3H6-SCR over iron catalysts supported on Aluminum Pillared Montmorillonite (Fe-Al-PILC)
Time : 14:40-15:10

Biography:
Yaxin Su received his Ph D of Power Engineering and Thermophysics with a focus on combustion from Zhejiang University, China, in 2000. He worked in the Department of Chemical Engineering, University of Mississippi, USA as a visiting professor during 2006-2007. He is currently a professor in the School of Environmental Science and Engineering, Donghua University, China.
Abstract:
Iron based catalysts supported on Aluminum Pillared Montmorillonite (Fe-Al-PILC) were prepared. The methods including XRD, H2-TPR, Py-FTIR, Uv-Vis spectroscopy, ICP, N2 adsorption-desorption, etc were used to characterize the basic physical and chemical properties of the catalysts. The characteristics of selective catalytic reduction of NO by propylene on the catalyst surface were studied experimentally in a fixed-bed reactor, and the effect of the Al3+/clay ratios on the physicochemical properties of the catalyst and the SCR-C3H6 was investigated. The results show that 9Fe/Al-PILC has higher SCR-C3H6 denitrification performance, e.g., 100% of NO conversion to N2 was tested over 400 °C. The Al3+/clay ratio plays more important role on NO conversion than the calcination temperature of the carrier. According to the Al3+/clay ratios, the order of catalytic activity is 9Fe/Al-PILC-10 > 9Fe/Al-PILC-20 > 9Fe/Al-PILC-5 > 9Fe/clay > 9Fe/Al-PILC-40. Al3+ increased the specific surface area of the montmorillonite dramatically, and the catalyst had micropores and mesoporous structures. When the Al3+/clay ratio was 10 mmol/g, the pillared montmorillonite had the best physicochemical property. In the Fe/Al-PILC catalysts, the iron oxides are highly dispersed on the surface of the support. H2-TPR shows that the reduction of Fe2O3 phase determines the SCR activity of the catalyst. With the increase of Al3+/clay ratio, the reducing temperature of the reducing process Fe3O4→Fe gradually increases. UV-vis results showed that Al3+ increased the oligomer FexOy, and the activity of the catalyst was positively correlated with the oligomer FexOy. Py-IR results showed that both Lewis acid and Bronsted acid were favorable for the selective catalytic reduction of NO. The 9Fe/Al-PILC-10 catalyst had the best activity, which was related to its higher Brønsted acid content.
Bharti Khungar
Birla Institute of Technology and Science (BITS) Pilani, India
Title: Design and Use of an Imidazolium Ion-tagged Fluorescent Chemosensor for Sensing Metal ions in Water
Time : 15:10-15:40

Biography:
Dr. Bharti Khungar is an Associate Professor and Head, Department of Chemistry, BITS Pilani, Pilani Campus, India. She carried out her doctoral research in Chemistry at University of Rajasthan, Jaipur, India and obtained the Ph.D. degree in 2002. She is working in the field green chemistry for synthesis, characterization and applications ion-tagged moieties. These ion-tagged molecules have been screened for biological applications, and catalytic properties on complexation with metal ions. Recently she is working for designing water soluble sensing material for detection of toxic metal ions in water.
Abstract:
In present scenario for the accurate detection of a contaminant in unprocessed environmental samples there is need to develop innovative chemosensors which provides excellent selectivity and sensitivity to heavy and transition metal ions. A major challenge of developing chemo sensors is the design of chelators possessing both high affinity and high selectivity. Most of the metal ions are carcinogens and lead to serious health concerns, hence, fast and accurate detection of metal ions has become a critical issue1. We have developed a simple fluorescent chemosensor containing only imidazolium ionic tag along with Schiff base for the detection of metal ion contamination in water. Ionic tags are entirely composed of ions, are drawing extensive interest, since they can be tailored to satisfy the functional requirements for building organic materials by changing either the cation or anion species 2. Ionic tags have achieved great success in acting as luminescent materials, exhibit strong fluorescence with high quantum yields 3. Schiff bases also are readily obtained by simple synthetic procedures and usually exhibit strong emission upon binding to specific foreign ions4. For this work 8-aminoquinoline is used as an amine source, which is a traditional fluorophore which is widely employed in the design of sensors due to its coordination function. Details of synthesis, characterization and sensing studies will be shown in presentation during the conference.
Vandana Khungar
University of Rajasthan, India
Title: Laws to Enhance Green Chemistry and Protect Humanity
Time : 16:00-16:30

Biography:
Vandana Khungar has completed her PhD in 2011 from the University of Rajasthan, Jaipur, India. Presently she is a Post Doctoral fellow from Indian Council of Social Science Research, New Delhi, India. She is working in the area of Indian constitution and laws.
Abstract:
With the development, human beings have created a background of destruction that necessitates incorporation of green chemistry in daily life. Adoption of this revolutionary and diverse discipline have led to significant environmental benefits, innovation and a strengthened economy. Laws have contributed significantly to enhance the concept of Green Chemistry in the United States. Toxic Substances Control Act (TSCA) of 1976 governs the majority of industrial chemicals, Pollution Prevention Act 1990 has helped foster new approaches for dealing with pollution by preventing environmental problems before they happen. In 2008, the State of California approved two laws aiming to encourage green chemistry, launching the California Green Chemistry Initiative. One of these statutes required California's Department of Toxic Substances Control (DTSC) to develop new regulations to prioritize "chemicals of concern" and promote the substitution of hazardous chemicals with safer alternatives.
Such laws should also be implemented in India to combat accidents like Bhopal gas tragedy. Due to the leak of gas in the world's worst industrial disaster in1984 at the Union Carbide India Limited (UCIL) pesticide plant, 500,000 people were killed due to methyl isocyanate and other gases. This was an incident of the past but a good example of how mankind is still struggling with the use of pesticides in the present is Cancer Train. The train commences from Abohar and leaves from the Bathinda station to reach Rajasthan's Bikaner, where patients undergo treatment at the Acharya Tulsi Regional Cancer Treatment and Research Centre (RCC). In Malwa (Punjab) farmers use 15 different pesticide sprays and the unregulated and excessive use of chemical fertilizers and pesticides have resulted in farmers and their families living in a cesspool of toxicity. Presently rise in cancer deaths (18/day) in Punjab can be attributed to indiscriminate use of agro-chemicals after the Green Revolution.
When developed nations like America can grow in the realm of green chemistry, then why not a developing nation like India?
When developed nations like America can grow in the realm of green chemistry, then why not a developing nation like India? The present study deals with recommendation of drafting and implementation of such laws in India and rest of the world, the details will be presented in the conference.
Jun ZHANG
Chinese Academy of Sciences, China
Title: High Value-added Cellulose Products Prepared from Low-grade Cellulose Resources
Time : 16:30-17:00

Biography:
Dr. Jun Zhang is a full professor of polymer science and materials at CAS Key Laboratory of Engineering Plastics, Institute of Chemistry, Chinese Academy of Sciences (ICCAS). He obtained his Ph.D. degree at Dalian University of Technology of China in 1999. He has published more than 150 research articles and three book chapters, and holds 28 china patents. His research interests include processing and functionalization of natural polymers, physics and chemistry of cellulose, ionic liquids and their applications in polymer materials, and high performance polymers and polymer composites.
Abstract:
Cellulose is the most abundant renewable organic material with a host of current and potential uses. Starting with dissolving pulp as a purified raw material, cellulose is converted industrially into regenerated materials (fibers, films, food casings, membranes, sponges, etc.) and cellulose derivatives (ethers and esters). However, until now, the main resource for commercial cellulose production are concentrating on the highly pure cellulose resources such as cotton linters and dissolving wood pulp. This fact makes the cellulose resource expensive to obtain. In contrast, the low-grade lignocellulosic biomass has become attractive as a renewable resource because it is available in large quantities and routinely widely cultivated in the world. At present, development of new and effective methods to convert low-grade cellulose into high value-added products is critical. With this aim, we developed two strategies: 1) all-cellulose nanocomposites reinforced with in-situ retained cellulose nanocrystals during selective dissolution of cellulose in an IL, and 2) blending low degree of polymerization (DP) cellulose with a small amount of high-DP cellulose. With these two strategies, some low-grade cellulose resources, such as agricultural straw, waste newspapers, and waste cellulose-containing fabrics, were converted into high value-added cellulose-based films. In this talk, I would like introduce our research progress in this field.
Konstantin I Galkin
N.D. Zelinsky Institute of Organic Chemistry RAS, Russia
Title: Catalytic dehydration of modified carbohydrates as a new approach to efficient biomass utilization in organic synthesis

Biography:
Abstract:
Chen Wang
North University of China, China
Title: The reliability study on the Cu/CHA NH3-SCR Catalysts: SO3 and Na ions poisoning

Biography:
Dr. Chen Wang is an associate professor of Environmental Engineering at North University of China, Taiyuan. He studied Chemical Engineering at Tianjin University and received his phD in this area from school of Chemical Engineering and Technology under the guidance of Dr. Wulin Wang, in 2017. After one year of postdoctoral research at Tianjin University with Prof. Meiqing Shen, he joined the department of Environment at North University of China. His research interests lie in the field of heterogeneous catalysis, mainly on the reliability research on Cu/CHA.
Abstract:
Part 1:
The deactivation mechanism of Cu/CHA ammonia selective catalytic reduction catalysts by SO3 poisoning has been systematically investigated using a range of analytical techniques. In order to study the influence of SO3 poisoning on active Cu2+ ions and the zeolite framework, different sulfate samples were prepared with different contents of SO3 (0 ~ 20%) in SOx under same poisoning condition. The results reveal the NO conversion of samples poisoned by SO3 decreased more than that poisoned by SO2 when temperature ranged between 100 oC and 600 oC. The TPR and EPR results demonstrate that SO3 poisoning does a significant influence on the amount of active Cu2+ ions than SO2 does. The kinetic results illustrate the SO3 poisoning has no impact on the apparent activation energy (Ea) of NH3-SCR reaction over Cu/CHA catalysts. The reason of NH3-SCR activity declining is the reduction of the number of isolated Cu2+ ions among the kinetic temperature regions. The ex-situ DRIFTs and BET results expose that the SO3 poisoning could decrease the crystallization by damaging Si-OH-Al structure. The NH3-SCR activity at high temperature decline because of the NH3 migration difficulty resulted by structure damaging.
Part 2:
Cu/CHA catalysts have been found to be affected by alkali and alkali earth ions; however, the poisoning mechanism is still unclear. In order to investigate Na poisoning effects and its mechanism on Cu/SAPO-34 and Cu/SSZ-13, five samples with different Na contents were synthesized. The Na effects on the structure, Cu species, and NH3-SCR reaction over Cu/CHA were characterized through XRD, BET, NH3-TPD, ex-DRIFTS, H2-TPR, EPR, activity tests and kinetic experiments, and CO2-DRIFTS were used to probe the types of Na species. The results indicate that the introduced Na+ exchanged with H+ and Cu2+, and it mainly substituted H+ from Si-OH-Al, then H+ from surface OH, finally isolated Cu2+. The exchanged H+ led to the structure damaging of Cu/CHA by dealumination, and the exchanged Cu2+ aggregated and formed CuOx species. The NH3-SCR activity decreased with Na contents, and the loss of isolated Cu2+ and CHA structure was responsible for the performance deactivation.

Biography:
Ksenija Kumrić has completed her PhD at the Faculty of Physical Chemistry, University of Belgrade, Serbia. She is employed at the Laboratory of Physics, "VinÄa" Institute of Nuclear Sciences, Belgrade, Serbia and engaged in the realization of the project "Physics and Chemistry with Ion Beams" financed by the Ministry of Education, Science and Technological Development of Republic Serbia. Research work is focused in the field of separation chemistry, especially membrane based separation processes and adsorption, and its application in radiochemistry, analytical and environmental chemistry.
Abstract:
Pesticides released from agricultural practices are an important class of pollutants due to their widespread use, toxicity, persistance, polar nature and water solubility. Monitoring of the pesticides comprises a sample preparation step - for separation and enrichment, and high performance techniques for quantifications. Solid phase extraction (SPE) has been proved to be an effective sample pretreatment method due to high enrichment efficiency, low consumption of organic solvents, simplicity and easy operation. The choice of appropriate adsorbent is a crucial factor to obtain high recoveries and high enrichment factors in SPE procedure. The aim of this study was to investigate the possibility of using activated carbon (AC) derived from coconut shell as the SPE packing material for the preconcentration of five varying polarity pesticides (imidacloprid, acetamiprid, carbendazim, simazine and linuron) from aqueous solutions before determination by high performance liquid chromatography with diode array detector (HPLC-DAD). The effects of the solution pH, eluent type, eluent volume and flow rate were investigated for optimization of the presented procedure. The adsorption was achieved quantitatively on AC column at the pH range 2.0 – 8.0, then the retained pesticides content was eluted with dichloromethane. Under the optimized conditions, the detection limit was found to be 29.3 - 121.4 ng/L, depending on the pesticide. The obtained results indicated that the proposed method could be used for the simultaneous determination of the varying polarity pesticides in environmental water samples at trace levels.

Biography:
Ksenija Kumrić has completed her PhD at the Faculty of Physical Chemistry, University of Belgrade, Serbia. She is employed at the Laboratory of Physics, "VinÄa" Institute of Nuclear Sciences, Belgrade, Serbia and engaged in the realization of the project "Physics and Chemistry with Ion Beams" financed by the Ministry of Education, Science and Technological Development of Republic Serbia. Research work is focused in the field of separation chemistry, especially membrane based separation processes and adsorption, and its application in radiochemistry, analytical and environmental chemistry.
Abstract:
Pesticides released from agricultural practices are an important class of pollutants due to their widespread use, toxicity, persistance, polar nature and water solubility. Monitoring of the pesticides comprises a sample preparation step - for separation and enrichment, and high performance techniques for quantifications. Solid phase extraction (SPE) has been proved to be an effective sample pretreatment method due to high enrichment efficiency, low consumption of organic solvents, simplicity and easy operation. The choice of appropriate adsorbent is a crucial factor to obtain high recoveries and high enrichment factors in SPE procedure. The aim of this study was to investigate the possibility of using activated carbon (AC) derived from coconut shell as the SPE packing material for the preconcentration of five varying polarity pesticides (imidacloprid, acetamiprid, carbendazim, simazine and linuron) from aqueous solutions before determination by high performance liquid chromatography with diode array detector (HPLC-DAD). The effects of the solution pH, eluent type, eluent volume and flow rate were investigated for optimization of the presented procedure. The adsorption was achieved quantitatively on AC column at the pH range 2.0 – 8.0, then the retained pesticides content was eluted with dichloromethane. Under the optimized conditions, the detection limit was found to be 29.3 - 121.4 ng/L, depending on the pesticide. The obtained results indicated that the proposed method could be used for the simultaneous determination of the varying polarity pesticides in environmental water samples at trace levels.
Radojka Vujasin
University of Belgrade, Serbia
Title: Use of waste sludge from Ni/Cr plating as raw material for obtaining the inorganic pigments

Biography:
Radojka Vujasin has completed her PhD at the Faculty of Physical Chemistry, University of Belgrade, Serbia. She is employed at the Department of Materials Science, "VinÄa" Institute of Nuclear Sciences, Serbia. As a researcher she is working in the field of materials science dealing with the synthesis, characterisation and modification of carbon materials and metal hydrides. She has experience in theoretical research using different computer programmes based on density functional theory.
Abstract:
Hazardous industrial waste is the most common source of environmental pollution. Waters originating from unregulated landfills and places of inadequate disposal of this type of waste can pollute the sources of drinking water and affect the health of the population. As a starting material for the synthesis of inorganic pigments, galvanic waste sludge from Ni/Cr plating was used. Color of the obtained inorganic pigments vary from green through brown to black, which can be attributed to the existence of Cr and Fe chromophore ions, as dominant components in the composition of sludge. Black inorganic pigment, mixed Fe/Cr oxide (Cr1.3Fe0.7O3), was synthesised by adding Fe2O3 to the sludge and choosing the appropriate temperature of calcination (1000 oC). Values of L*a*b* coordinates were 23.8, 1.6 and 0 were obtained respectively and are in accordance with the literature. XRD, SEM, PSD (particle size distribution) analysis were performed on the as received sludge and synthesized pigment. Black pigments can be considered as “inert”, against the water leaching, which is quite opposite to the leaching stability of the dried sludge.
Ljiljana Matović
University of Belgrade, Serbia
Title: Synergetic effect of ionizing radioation and adsorption on methylene blue degradation

Biography:
Ljiljana Matović is a director of Department of Material Science, "VinÄa" Institute of Nuclear Sciences. As a researcher she is working in the field of Material Science, dealing with the synthesis, characterization and modification of different kind of materials ranging from synthetic (carbon, metal hydrides) to natural (clay, zeolites) and their composites. The main field of interest are materials for waste conversion. She has teaching experience (as a mentor) of PhDs and Masters theses as well as management experience (as science project leader) and experience in production and investigation of radiopharmaceuticals.
Abstract:
Dyes and pigments are used by many industries to color their products. Presence of dye molecules, even at very low concentrations, is undesirable and may significantly affect photosynthetic activity in aquatic systems. A new approach that combines the use of carbon material, as an adsorbent matrix, coupled with the high energy irradiation derived from the radioactive waste sources was used for degradation of Methylene blue (MB), which was taken as an example of contaminant. Synergistic effect on the degradation of the dye was investigated. Irradiated solutions of MB with carbon-based material show significantly greater decrease in absorption then the unirradiated solutions using only carbon-based material or irradiation. Positions of all bands in spectra remain unchanged except a slight shift and changes in the relative intensities of the bands at 1100 cm-1 assigned to C-O stretching in phenolic groups (1000-1250 cm-1). High energy radiation in water medium can produce radiolysis of water i.e. several active species such as H2, H2O2, H+, OH−, eaq−, •OH and •H. As a result of formed reactive speaces which originate from the irradiated material and radiolysis of water, the amplitude of all absorption bands characteristic for MB disappeared completely. Joint application of those techniques, radiolysis and adsorption, using waste radioactive sources, as well as modified waste materials, has not been applied before.
Rong-Xin Yuan
Changshu Institute of Technology, China
Title: 3D Water-Stable Magnesium Metal-Organic Framework for Sensoring Fe3+Ion

Biography:
Rong-Xin Yuan has completed his PhD in 2002 at Nanjing University.During 2002-2004, he worked at University of Bielefeld and University of Nottingham as postdoctoral fellow. Now he is the director of Key Lab of Advanced Functional Materials. He has published more than 80 papers in reputed journals.
Abstract:
Excessive Fe3+ ions in water will cause great harm to the human body, even if it is required. Therefore, the design of new flurescence probes for Fe3+ ion is very important . Herein, Mg(HPCD) (H2O) (H3PCD=9-(2-(ethoxy(hydroxy)phosphoryl)ethyl)-9H-carbazole-3,6-dicarboxylic acid) was synthesized and characterized. Adjacent {MgO6} octahedra are joined by O-P-O and O-C-O groups into 1D chain, which are linked by the HPCD2- ligands to form a 2D layer parallel then packed to form a 3D network(Fig.1). The Fe3+ ion can reduce fluorescence relative to other metal ions (Cd2+, Co2+, Cu2+, Sr2+, Al3+, Zn2+, Mn2+, Pb2+, Fe2+) (Fig.2), so the compound may be used as a fluorescence prob for Fe3+ ion.
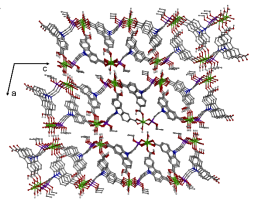
Fig.1 Structure of Mg(HPCD)(H2O) viewed along the b-axis.
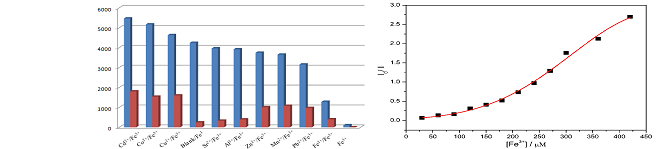
Fig.2 Fluorescence intensities of Mg(HPCD)(H2O) immersed in the individual aqueous solutions
Marta Pineiro
University of Coimbra, Portugal
Title: Development of a cellulose based biodegradable material for packaging

Biography:
Marta Pineiro achieved her Ph.D. in Organic Chemistry in 2003 in the University of Coimbra, Portugal, and since 2002 has been enrolled at the University of Coimbra, being at present Auxiliary Professor. Her research interests are in the area of sustainable synthesis, microwave-assisted organic synthesis and synthesis and biological applications of tetrapyrrolic macrocycles. She has published so far 50 articles in international peer-reviewed journals, 17 meeting proceedings and is the co-author of 6 books and book chapters and 4 patents.
Abstract:
About 38% of the 407 millions tons of plastics produced worldwide is used in the packaging industry in a market dominated by the polyolefins (PP,LDPE,HDPE). This means that, only by this activity, 154 millions tons of waste are generated and a huge part of this will be lost from any recycling circuit finishing their brief lives in landfills[1]. The introduction of biodegradable materials in packaging industry will reduce the pressure over the deposition in landfills but the decomposition must be in a way that safe products are generate during the process of environment friendly biodegradation to carbon dioxide [2].The non-edible nature of cellulose, its abundance as available material in the form of wood or agriculture residues and its renewable and biodegradable characteristics makes this natural polymer an interesting material to be used as environmental friendly packaging materials.[3] However, cellulose lacks thermoplasticity which means that it must be blended with other polymeric materials in order to get possible to be workable in packaging industry. Polyesters are generally biodegradable polymers due to the reversibility of the ester bond by hydrolysis. The manipulation of the structure could turn the mechanical properties of the polyesters more similar to polyolefins and in a future replace them as a main thermoplastic source. Actually polyesters are more expensive than polyolefins but the introduction of low cost fillers could reduce costs and improve mechanical properties [4].
In this work we present the results of the combination of hydrolysed pulp cellulose with Bioflex® which a mixture of two biodegradable polyesters polylactic acid (PLA) and polybutylenesuccinate (PBS). The mechanical behaviour of composite samples with different amounts of cellulose will be discussed.
Tofik Nagiev
Nagiev Institute of Catalysis and Inorganic Chemistry, Azerbaijan
Title: Mechanism of Biomimetic Monooxidation of Cyclohexane by Hydrogen Peroxide

Biography:
Tofik Nagiev is a Vice-president of Azerbaijan National Academy of Sciences, Director of Research Center of “Azerbaijan National Encyclopedia” and Department chief of Nagiev Institute of Catalysis and inorganic chemistry of ANAS. The Professor of the department of the physical and colloid chemistry of Baku State University. His main publications are listed below:
- M.F.Nagiev and T.M.Nagiev "The Conjugate Dehydrogenation of Hydrocarbons", Book: Advances in Chemistry-133, Editor Hugh M. Hulbert, 1974. pp. 137-147, USA.
- Tofik M. Nagiev. Coherent Synchronized Oxidation reactions by Hydrogen Peroxide. Elsevier. Amsterdam. 2007, p.340. Monography
- T.M.Nagiev. Chapter. "Physicochemical Peculiarities of Iron Porphyryn-Containing Electrodesign Catalase and Peroxidase-Type Biomimetic Sensor "Book" Biomimetic Based Applications", Preface IX, Chapter4, 2011, p.105-123 under the editorship of Anne George, Croatia.
Abstract:
The inducing effect of hydrogen peroxide on synchronous monooxidation reaction of hydrocarbons in the presence of biomimetic catalysts is accompanied by two interrelated and interacting - coherent reactions. The decomposition reaction of H2O2 (primary) forms a biomimetic catalytic intermediate, in the interaction of which with the substrate, its transformation occurs in a secondary reaction-coherently synchronized with it.
The mechanism of such coherent-synchronized reactions is considered in the process of heterogeneously catalyzed monooxidation of cyclohexane by hydrogen peroxide in the gas phase in the presence of a biomimetic catalyst, which is described by the following generalized scheme:

It follows from this scheme that the primary H2O2 decomposition reaction forms highly active hydroperoxide active center ImtOOH, which interacts with cyclohexane by forming the desired products (secondary reactions) - C6H11OH, C6H10O, C6H10 and C6H8.
As a biomimetic catalyst was used per-FTPhPFe(III)OH/Al2O3, which synthesized on the basis of the iron-porphyrin complex, simulating the catalytic functions of the enzyme of the oxoreductase-catalase and monooxygenase group, which are distinguished by their selective and highly active action and necessary for the creation of effective biomimics.
Experimental study of the monooxidation reaction of cyclohexane shows that the process of formation of cyclohexanone, cyclohexanol and cyclohexene proceeds along a sequentially parallel mechanism, which can be represented as the following scheme:
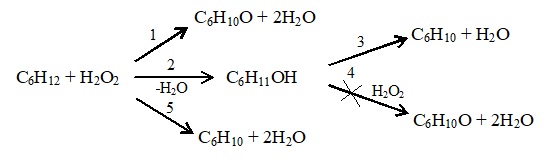
Each stage indicated in this scheme proceeds according to the previously described coherently synchronized mechanism.
Carla Gomes
University of Coimbra, Portugal
Title: Mechanochemistry for two-step synthesis of porphyrins

Biography:
Carla Gomes research interests are in the area of sustainable synthesis, microwave-assisted organic synthesis and mechanochemistry specially related to the synthesis of heterocycles. She has presented three orals communications and three posters with two of them winning the best poster prize.
Abstract:
The remarkable versatility of porphyrins and their derivatives, clearly demonstrated by the large number of applications, relies in a great length on the development and improvement of synthetic strategies that, over the years, made possible the huge availability of these compounds. The synthesis of meso-substituted porphyrins from pyrrole and aldehyde involves the cyclization to obtain the porphyrinogen and the oxidation to aromatize it to porphyrin. With the awakening for the environmental issues and the establishment of Green Chemistry began the search for new synthetic methodologies often using new tools such as microwave or mechanochemistry. In this communication we report the synthesis of porphyrins using mechanochemistry. The influence of the ball milling process in the cyclization and oxidation step, the acid catalyst, the oxidant, the substitution at the aldehyde and the presence and type of solvent will be discussed.
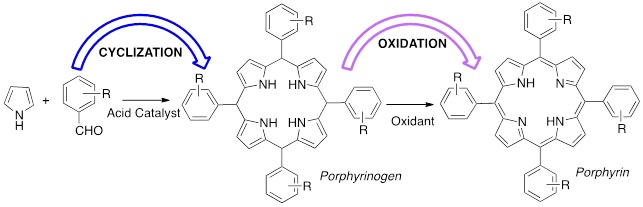
Haley Irving
Portland State University, USA
Title: Computational characterization of a visible light sensitized tellurorhodamine catalyst for thiol oxidation

Biography:
Haley Irving completed their B.A. in Chemistry at Pitzer College, of the Claremont College Consortium in Los Angeles California and is currently enrolled in the chemistry PhD program at Portland State University with the McCormick Photochemistry group. Haley expects to graduate with a PhD in 2021. Haley’s current research involves coupled experimental and computational characterization of tellurorhodamine photocatalysts for aerobic oxidation catalysis. Haley is also acting as Vice President of the PSU Women in STEM.
Abstract:
Homogeneous metal-catalyzed aerobic oxidation reactions have been of increasing interest due to thepotential in green industrial applications. However, some industrially implemented methods require stoichiometric amounts of halogenated sacrificial electron acceptor to oxidize the Mn to M(n+2), the active oxidant species. Organochalchogen dyes, and more specifically tellurium-containing dyes, have been demonstrated to photocatalytically oxidize thiols without the use of chemical oxidants. Computational modeling coupled to experimental results allows further understanding of oxidation and reduction mechanism associated with this catalytic cycle. This reaction is catalyzed by a visible-light-active, self-sensitized, tellurium-containing chromophore under ambient temperature and pressure. The tellurium-containing chromophore is a rhodamine-derivative that is photo-oxidized to the active telluroxide species without the addition of a photosensitizer or external oxidant (besides atmospheric oxygen). The mechanism of telluroxide formation and thiol to disulfide transformation were determined computationally and are supported by results from stop-flow spectroscopy experiments.
Chung-Hsin Wu
National Kaohsiung University of Science and Technology, Taiwan
Title: Hydrothermal synthesis of novel BiVO4/Ag3VO4 composite with high visible-light-induced photocatalytic activity

Biography:
Chung-Hsin Wu received a Doctorate in Environmental Engineering from National Taiwan University in 1999 and Dr. Wu joined the faculty of Chemical and Materials Engineering at National Kaohsiung University of Science and Technology in 2010 as a Professor. Prof. Wu’s research interest includes treatment processes for contaminated waters and hazardous chemicals, for which seven Taiwan patents have been issued. Prof. Wu is currently working on the synthesis of novel photocatalysts. Prof. Wu has reviewed over 300 papers submitted for publication to various journals and he has authored over 100 journal papers and 80 conference presentations and seminars.
Abstract:
A hydrothermal process is utilized to synthesize silver orthovanadate (Ag3VO4, AVO), bismuth orthovanadate (BiVO4, BVO) and BiVO4/Ag3VO4 composite (BVO/AVO). The precursors of AVO were silver nitrate (AgNO3) and sodium orthovanadate (Na3VO4), and those of BVO were bismuth nitrate (Bi(NO3)3) and ammonium metavanadate (NH4VO3). The Bi/Ag molar ratio in the BVO/AVO preparation was 1/3. The effects of heating time (6, 12 and 24h) and temperature (393, 433 and 473K) on the synthesis of AVO were elucidated. C.I. Reactive Red 2 (RR2) was used as the parent compound in evaluating the photocatalytic activity of all prepared photocatalysts. The optimal experimental conditions for synthesizing AVO by the hydrothermal method included initial heating at 393 K for 6 h. A 400 W Xe lamp was used as the light source. A quartz appliance that was filled with 2 M NaNO2 solution was placed on the top of the photo-reactor to filter out the ultraviolent (UV) and to provide visible-light (Vis). The rates of RR2 photodegradation by all photocatalysts under UV and Vis irradiation followed a pseudo-first-order kinetic model. The RR2 photodegradation rate constants in the UV/AVO, Vis/AVO, UV/BVO, Vis/BVO, UV/BVO/AVO, Vis/BVO/AVO and solar/BVO/AVO systems were 1.43, 0.03, 0.55, 0.16, 5.06, 2.39 and 2.48 hr-1, respectively. BVO/AVO exhibited the highest photocatalytic activity of all the prepared photocatalysts. This investigation suggested that the coupling of BVO greatly inhibited the recombination of photo-generated electron-hole pairs in AVO, revealing that the separation of photo-generated electron-hole pairs in BVO/AVO was more efficient than that in AVO. Adding isopropanol to the RR2 solution slightly reduced the rate of RR2 photodegradation but adding Cr(VI) and EDTA-2Na markedly slowed RR2 photodegradation. The experimental results suggested that the photogenerated holes and superoxide radicals were the main oxidative species for RR2 photodegradation in AVO and BVO/AVO systems.
Yuji Fukmoto
Kindai University, Japan
Title: Production of antifungal active substance using Biofilm by Bacillus subtilis
Biography:
Abstract:
World population is now increasing, and the United Nations predicts that the world population will be 9.8 billion by 2050. As a result, higher food production is required. One of the solutions is the use of pesticides. In order to achieve higher food production, the burden on the environment has to be smaller and more sustainable. In view of this fact, microbial pesticides have attracted attention in recent years. Microbial pesticides are agents that use cells and/or the substances produced by microorganisms and inhibit the growth of phytopathogenic fungi. Compared with chemical pesticides, microbial pesticides are less likely to remain in the environment, and it is difficult to develop drug-resistant bacteria. Therefore, we focused on Bacillus bacteria in this study. Bacillus bacteria are broadly distributed microbial microorganisms in the soil. It is reported that bacteria of the genus Bacillus form spores and biofilms and are resistant to growth inhibitory conditions. Using Bacillus subtilis strain RB14, which is known to produce iturin A, an antifungal substance, we examined the influence of medium concentration on biofilm formation and the production of antibiotic substance. The relationship between biofilm formation and antifungal substance production was clearly observed, and it was shown that antifungal substance was produced after biofilm formation. We have previously observed the biofilm formation in the medium with the agriculture residues. Using these properties, the experiment to increase the production amount of antifungal substance is under consideration.
Minori Maeda
Kindai University, Japan
Title: Basic analysis of volatile organic compounds from actinomycetes
Biography:
Abstract:
In recent years the food problem has become serious as the world population increases. As a consequence, further productivity improvement is required in agriculture. The control of phytopathogens which adversely affect crops, in particular, is indispensable for stable crop production. Currently, chemical pesticides are mainstream as a means to do so, but also microbial pesticides having various effects are attracting attention. As a candidate of microbial pesticide, our laboratory has isolated actinomycetes with antimicrobial activity against multiple phytopathogens. These actinomycetes produce gaseous antimicrobial substances. In order to gain insights on Volatile Organic Compounds (VOCs) derived from actinomycetes, we focused on actinomycetes AR3, AR4 and AR10 strains which were found to be effective in the infection control experiments. First, the effects of VOCs derived from actinomycetes on growth of phytopathogens were investigated. actinomycetes spore suspension was applied to the surface of the PDA medium and cultured for 3 days. After that, the petri plate with agar pieces of phytopathogen was inverted on top of the plate with actinomycetes and both plates were sealed and cultured for several days. As a result, the growth of phytopathogens was suppressed. In addition, VOCs derived from actinomycetes were subjected to GC-MS analysis. An adsorbent was placed inside a petri dish coated with actinomycetes and incubated for 3 days to adsorb VOCs. The adsorbent was extracted, and subjected to GC-MS analysis. As a result, very similar peaks were detected in AR3 and AR4 strains. We are currently trying to identify VOCs, including analysis of AR10 strain.
Hiroshi Yukimoto
Kindai University, Japan
Title: Enhanced performance of soil microbial fuel cell by earthworms
Biography:
Abstract:
Currently, energy supply source from fossil fuels have ploblems such as depletion of petroleum and greenhouse gas emissions. Under the circumstances, demand for renewable and sustainable energy is increasing and the alternative energy sources other than fossil fuels are expected. Soil microbial fuel cells (SMFCs) are devices that using microbes in the soil as biocatalysts to convert chemical energy to electricity. These are expected as an application to produce sustainable energy. But it is still a long way to go before SMFC is practically applied. One of the problems is low power generation by SMFC operation. Here, we focused on soil ecosystems, specifically the earthworms which are known to improve soil-fertility by degrading fallen leaves or plant litter and we investigated the effect of earthworm on power generation of SMFC. Earthworms were added to SMFC and the maximum power density and the internal resistance were compared to SMFC with and without earthworms. As a results, The power density increased by 800% and the internal resistance decreased by 91.5%. The soil structure of SMFC with and without earthworms was different and the clear soil aggregate structure was found in SMFC with earthworms, which had been made with the passage of soil through the earthworm gut. The results indicated that adding earthworms had a significant effect on the SMFC performance, especially the power and soil structure, and it is suggested that this system would contribute to the sustainable and rewewable energy source.
- Green Chemistry and Technology | Green Chemical Engineering | Environmental Chemistry and Pollu on Control | Green Analytical Chemistry
Location: Meeting Place 2

Chair
Dimitris S. Argyropoulos
North Carolina State University, USA

Co-Chair
Ricardo Reis Soares
Federal University of Uberlandia, Brazil
Session Introduction
Ricardo Reis Soares
Federal University of Uberlandia, Brazil
Title: Fatty acids, olefins and green diesel from catalytic edible oils hydrolysis
Time : 11:40- 12:10

Biography:
Prof. Ricardo Soares has completed his PhD in 1997 at UFRJ/Brazil and postdoctoral studies from Oklahoma University and University of Wisconsin, USA. He is the coordinator of the Biofuels Graduate Program at Federal University of Uberlandia, Brazil. He has published more than 25 papers in reputed journals and has been working with several brazilian industries, such as PETROBRAS and CBMM.
Abstract:
Studies using heterogeneous catalysts for the hydrolysis reaction of edible oils still are in early stage. Recently, it was found that the aqueous solution of glycerol, formed after the hydrolysis, may suffer the reaction of aqueous phase reform (APR) producing H2 and CO2. The H2 can be used in the hydrogenation of unsaturated free fatty acids formed, allowing to obtain a specific fatty acid of higher added value. The present work demonstrates that you can tune in the final product by choosing the appropriate sequential catalyst system as shown in the figure below.
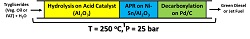
ZHANG Feng
Chinese Academy of Inspection and Quarantine, China
Title: Green Analytical Techniques in Food Analysis
Time : 12:10-12:40

Biography:
Prof. Dr. Feng ZHANG, was born in 1974 in Shandong province, China, Director of the Institute of Food Safety in Chinese Academy of Inspection and Quarantine, Professor of Xi'an Jiaotong University, the winner of funding of Max-Planck Society. Prof. Dr. Feng ZHANG returned to China in 2006 and focused his attention on the application of chromatography/mass spectrometry in food and drug analysis, measurement and standard material development and other fields. Dr. ZHANG has published more than 100 papers in peer-reviewed journals and 6 books, authorized 9 patents and established 6 national standards. In the recent years, as the project leader, he has undertaken more than 30 research projects and has obtained many research awards from government and national research association.
Abstract:
The development of greener analytical techniques is a topic of great interest. There is an increasing need for new analytical methods that can be used for assuring safety and quality in food samples, including adulterants, pesticide residues and unknown functional components. Ultra performance convergence chromatography (UPC2) is considered a valuable tool helping to separate and determine compounds differing by subtle structural differences. UPC2 presents several advantages over high performance liquid chromatography (HPLC), it takes less column equilibration time and consumes less organic reagents. UPC2 has recently been successfully used to separate and determine a lot of analytes, including many pharmaceutical compounds.
In this work, recent applications of UPC2 for the analysis of different compounds in food and biological samples were reviewed, in the hope of helping chromatography users to have a new look on the possibilities offered by this technique. Furthermore, a simple, highly sensitive and fast analytical method based on UPC2 with photo-diode array detection (PDA) has been developed to quantify quantify sulfonamides, monosaccharide, and structural analogues of isoflavones isomer in food. Additionally, authentication technology based on fragment markers and high resolution mass spectrometry was developed for the quality assurance and pesticide residues compounds analysis in food.
Anet Režek Jambrak
University of Zagreb, Croatia
Title: Green solvents selection for high voltage electrical discharge extraction of bioactive compounds form S. officinalis
Time : 12:40-13:10

Biography:
ANET REŽEK JAMBRAK, Associate professor is working at the Faculty of Food Technology and Biotechnology of the University of Zagreb, Croatia. She is working in the area of nonthermal and advanced thermal processing techniques, food chemistry, food physics, and process engineering. She also has strong international collaboration with renowned scientists. In the period from 2007. Anet Režek Jambrak has published over 80 significant scientific papers, published in top scientific journals with high impact factors (citation more than 1300, h-index 20). She is the winner of the 2016. Young Scientist Award from the International Union of Food Science and Technology.
Abstract:
The use of "green" solvents is driven by trends that are focused on finding solutions that minimize the use of solvents or find alternatives. Using the above mentioned solvents is directed towards intensifying the process of extraction and cost-effective production of high-quality extracts. The focus of the study was to use natural and bio-derived solvents in applications. Our goal was to develop, implement and promote the implementation of safer, greener technologies and sustainable industrial solvents. The focus was to select best solvents among water and bio solvent (polar, non-polar, protic and aprotic), including terpene, vegetable oil, MeTHF, NADES (natural eutectic solvent) and Switchable Solvents (Variable solvent) to extract bioactive compounds from sage (S. officinalis). Model predictive tools (Hansen, COSMO-RS) was used to predict the properties and behaviour of the interaction of solvent-solute, and to predict the most favourable performance of these solvents for targeted compounds. The extractions of 1g of sample in 50mL of solvents (water and ethanol) were achieved by high voltage electrical discharge device (IMP-SSPG-1200, Impel group, Zagreb, Croatia). Extraction were made using range of voltage from 15kV-25kV, at 100Hz frequency, during 3 and 9 min treatment time, using Argon as working gas. In this study bioactive compounds (α-thujone and camphor) from sage (S. officinalis) were chosen for COSMO-RS and HANSEN calculations for the selection of green solvents for high voltage electrical discharge-plasma extractions. The best solvents for extraction predicted by COSMO-RS are ethanol, ethylacetat, methylacetat, CPME, DMC, MeTHF.
Weiguo Cheng
Chinese Academy of Sciences, China
Title: Ionic Liquid-based Green Process Engineering for Coproduction of Ethylene Glycol and Dimethyl Carbonate
Time : 14:10-14:40

Biography:
Weiguo Cheng has completed his PhD from Dalian University of Technology in 2005. He has been professor of chemical engineering at Institute of Process Engineering, Chinese Academy of Sciences since 2014. He is the Member of Committee of Integration of IT Application and Industrialization. He has published more than 50 papers in reputed journals and and 19 invention patents granted including 1 PCT patent.
Abstract:
Ethylene glycol (EG) as one of bulk chemicals and dimethyl carbonate(DMC) as environmental friendly chemical material have strategic significance for the basic industries and new industries. The development of green process engineering for coproduction of EG and DMC is highly required as the conventional routes involve either high energy consumption or toxic material. The successful industrial ionic liquid-based green process engineering has several advantages. First, the supported ionic liquid based on synergistic catalytic effect in a fixed bed avoids energy consumption and the loss of catalyst, compared with traditional processes in which the separation of ethylene carbonate and catalyst cost a lot of energy. Second, reactive distillation breaks the equilibrium of the transesterification reaction and converts all ethylene carbonate, thereby improving conversion. This is more straightforward than using a series of fixed bed reactors and ethylene carbonate hydration reactor. Third, energy consumption is reduced (20% ~ 30%) due to the heat integration system compared with traditional process of the coproduction of propylene glycol and dimethyl carbonate. Finally, CO2 released from the upstream of ethylene oxidation plant is utilized in the new process, a big advantage from an ecological point of view, compared with traditional process of the hydration of ethylene oxide. This green chemical engineering technology has been pushed to commercialization. A 33,000 t/a industrial plant is now successfully operating. Because of its economic cost and environmental benign, the new process is believed to be a competitive technology for producing ethylene glycol and dimethyl carbonate.
Morteza Ziyaadini Avarani
Chabahar Maritime University, Iran
Title: Optimization of Reverse Phase Liquid-Liquid Microextraction (RP-DLLME) Method Coupled with High Performance Liquid Chromatography (HPLC) for the determination of chlorophenols (CPs) in marine sediments
Time : 14:40-15:10

Biography:
Morteza Ziyaadini Avarani has completed his PhD at the age of 31 years from University of Sistan & Balouchestan. He is a scientist who works at Chabahar Maritime University. The field of his research interest is the Marine Sciences and Marine Chemistry. He has published more than 15 papers in reputed journals.
Abstract:
The analysis of Chlorophenols (CPs) from environmental samples is an important topic because of their effects on the estrogen's health of humans and wildlife. Sediments or solids are good adsorbents of phenolic pollutants due to their active and extensive adsorbent and superficial surface activity. Sediments can accumulate this material with high concentrations and affect aquatic life. Due to the importance of monitoring the analysis of phenolic compounds in sediment and solid samples, it has been widely studied. Especially in this study, a quick, simple and inexpensive method is used to measure CPs in marine sediments. The reverse phase liquid-liquid microextraction (RP-DLLME) method was used to pre-concentrate of CPs after initial extraction by extraction of ultrasound waves measured by HPLC apparatus. Factors such as extraction time, pH, time and speed of centrifugation, type and volume of extraction solvent and Effect of the volume of disperser solvent were optimized. Under optimal conditions linear ranges for 2-chlorophenol and 2-4-dichlorophenol were between 0.001-2 mg.Kg-1, and 0.2-2 mg.Kg-1 respectively. The concentration factor of 101 and 102 and the relative standard deviation (n=5) 5.9, 3.3 were obtained for 2-chlorophenol and 2,4-dichlorophenol, respectively. Then suggested method has been used for determination of CPs and 0.21-2.18 mg.Kg-1 as well as 0.68-2.55 mg.Kg-1 values were determined for 2-chlorophenol and 2-4-dichlorophenol respectively in marine sediments of Chabahar Bay.
Ibrahim A. Salem
Tanta University, Egypt
Title: Homogeneous and Heterogeneous Catalytic Oxidation of Some Azo Dyes using Copper(II) ions
Time : 15:10-15:40
Biography:
Prof. Ibrahim Salem is a former Acting president and Vice president for post-graduates & research affairs of Tanta University, Egypt. He is the professor of Physical Chemistry at the Chemistry Department, Faculty of Science, Tanta, Egypt and he published 42 international publications in highly reputed journals. His main research interest is the study of the kinetics of environmental catalytic processes.
Abstract:
The kinetics of the homogeneous and heterogeneous catalytic oxidation of three azo dyes in presence of copper ions / copper ions – supported zinc oxide was investigated in aqueous solutions. These dyes were Chromotrope 2B (C2B), Chromotrope 2R (C2R) and Chrysophenen (CRY). The reaction progress was followed by monitoring the decrease in absorbance at â„·max equals to 512, 511 and 401 nm respectively. The rate of reaction increased with increasing either the concentration of the dye and the catalyst, giving a plateau at high concentrations. On the other hand, the rate of reaction increased gradually with increasing hydrogen peroxide concentration attaining a maximum then decreased thenafter. The reaction rate was also increased with increasing pH and temperature and was found to be entropy controlled.
Arvind Kumar
CSIR-Central Salt and Marine Chemicals Research Institute, India
Title: All Ionic Liquid Emulsions: Novel Green Recyclable Reactors for Synthesis of Nano- and Hybrid Materials

Biography:
Dr. Arvind Kumar did PhD in Chemistry at Kurukshetra University, India currently serves as Principal Scientist & Head, Salt and Marine Chemicals Division at CSIR-Central Salt & Marine Chemicals Institute Bhavnagar, Gujarat. His research interests cover design and development of ionic liquids and their use in green chemistry for sustainable technology. He visted Germany under a DAAD Fellowship-2004-05, and USA, under a CSIR-Raman Research Fellowship 2011-12 and is recipient members of the CSIR Rural Technology Award-2008. He has published over 100 research papers, holds several patents, book chapters and articles in popular magazines.
Abstract:
Room temperature ionic liquids (RTILs) are the organic analogues of inorganic molten salts with melting temperature < 100oC.[1] Being ionic in nature, these compounds are versatile in terms of solvent properties such as low volatility, high thermal stability, wide liquid range and good solvating ability. In view of flexibility of choice of cations or anions, RTILs can designed as low viscosity media suitable for self-assembling of amphiphile molecules or also can also be designed as surfactants by incorporating amphiphilic character in either cation or anion or in both the constituents. Therefore, with extraordinary properties it has been possible to include RTILs as media or as a surfactant or both for construction of colloidal formulations/self-assembled structures. Such structures are highly thermally stable and can be used as templates for many applications such as nanoreactors for preparation of shape/size controlled nanomaterials/quantum dots and their hybrid materials for applications such as novel light harvesting materials with enhanced quantum efficiency. The presentation will focus on construction of a novel all ionic liquid based microemulsion system, its characteristics and application for materials preparation in a recyclic manner.
Mariana Costa
Instituto Politécnico do Cávado e do Ave – IPCA, Campus do IPCA, Portugal
Title: Development of novel autoreactive and ecological monocomponent adhesives
Biography:
Abstract:
The growing concern about environmental issues has become a pressing issue for businesses as the applicable legislation requires more control of any type of hazard that puts human health or the environment at risk. Currently, chemicals are an important aspect of risk assessment and monitoring because of their importance as constituents of the raw materials and their effects on human health and / or the environment.
The aim of this project is developed a microencapsulation device, with reduced content of COVs and reduced risk for Health and Environment. In this work, we report a microfluidic approach, to fabricate monodisperse isocyanate microcapsules with lipophilic cores and polyurethane shells. These microcapsules are generated in a microcapillary microfluidic device we designed using monodisperse O/W emulsion, with high potential application in aeronautic and automobile industries.
The proposed method has advantages of being readily controlled, cost-effective and easy to operate, together with its ability to produce a uniform size. In addition, microfluidics can control the process of encapsulation by varying flow parameters and/or using a proper geometry of microfluidic channels. By microencapsulating the reactive agent, the product is safer for handling by the industry operators, and the activation mechanism can be controlled more precisely (enabling higher flexibility of application / use case scenarios).
The advances made of the current study can be an important contribution in the innovation and development of new methods and products sustainable/green that can, in the future, compete in the market.
Fares D Alsewailem
King Abdulaziz City for Science and Technology, Saudi Arabia
Title: Date pits: efficient fillers for polymers
Biography:
Abstract:
Date pit, a byproduct of the palm trees (Phoenix dactylifera L.) with a less value, was successfully used as efficient and eco-friendly filler and reinforcement for polymeric materials. Polystyrene (PS) was used as a matrix to host date pits granules. PS pellets were melt compounded with proper amount of date pit granules using extrusion technique. Morphological and mechanical properties of the prepared composites were investigated to explore effect of date pit particle size on the properties of the composites. It was found that mechanical properties represented by Izod impact strength may be significantly enhanced by controlling date pit particle size (in the range of 0.25-1.00 mm). One potential application of this product is thermal insulation sheets in buildings.
Shilpa Sudhakar Harak
M.S.Gosavi College of Pharmaceutical Education & Research, India
Title: Indian Aloe as an aid for storage of fresh fruits and vegetables

Biography:
Dr.Shilpa Sudhakar Harak is a doctrate in Pharmaceutical Chemistry from the Savitribai Phule Pune University. She is a keen academician and researcher. Till recent times, her research was focused on synthetic chemistry for which she has 5 patents published under Indian Patent Act and five research papers in reputed journals. She has now ventured in the field of green alternatives in supporting the cause for environment protection.
Abstract:
Hydrocarbons which are used in refrigiration units have been banned and instead their cousins the hydrochlorofluoro carbons are being used highly and still pose a danger to the depleting ozone layer. The ozone layer protects the earth from certain harmful ultra-violet radiations. However for several major reasons, refrigeration has no alternatives, viz. storage of fresh foods like fruits and vegetables.
Aloe vera (indian aloe) was found, identified and collected. The translucent properties of aloe vera indicated that applying a layer of aloe vera gel to the surface of the fresh fruits and vegetables restored the freshness for long time periods (upto 60 days) and prevented microbial growth during the storage period. Microbial studies of these samples done periodocaly indicated growth rate of microbes to be minimal. (no perishing) Also moisture studies have acertained the minimal loss of moisture in comparison to the storage at room temperature and refrigiration. Also physical analysis for skin colour, texture and eruption showed results comparable to refrigerated products. Developing this on a large scale will enable us to reduce the cold storage units employed for storage of fresh fruits and vegetables all over the world. This would reduce the amount of HCFCs being released in the stratosphere and thus benfit mother EARTH and her children.
Babatunde Oluwole Ogunsile
University of Ibadan, Nigeria
Title: Biosynthesis of Silver Nanoparticles from Leaves Extract of Tithonia diversifolia and Its Anti-microbial Properties

Biography:
Dr B. O. Ogunsile has completed his PhD at the age of 37 years from University of Ibadan, Nigeria and a Visiting Researcher at Universidad PontificiaBolivariana (UPB), Colombia and Indian Institute of Technology Roorkee(IITR), India. He is Associate Professor (Industrial/Material Chemistry) at the Department of Chemistry, University of Ibadan. His area of interest includes pulping potentiality of non woods, biosorption and remediation of dyes and metals, natural fiber reinforce composites and nanoscale particles and materials. He has published more than 34 papers in reputed journals and has been serving as a post-graduate science board member at the University of Ibadan, Nigeria.
Abstract:
High costs and toxicological hazards associated with the physico-chemical methods of producing nanoparticles have limited their wide spread use in clinical and biomedical applications. An ethically sound alternative is the utilization of plant bioresources as a low cost and eco –friendly biological approach. Silver nanoparticles (AgNPs) were synthesized from aqueous leaf extract of Tithonia diversifolia plant. The UV-Vis Spectrophotometer was used to monitor the formation of the AgNPs at different time intervals and different ratios of plant extract to AgNO3 solution. The biosynthesized AgNPs were characterized by FTIR, X-ray Difraction (XRD) and Scanning Electron Microscope (SEM). Antimicrobial activities of the AgNPs were investigated against ten human pathogens using agar well diffusion method. The AgNPs yields were modelled using a second-order factorial design. The result showed that the rate of formation of the AgNPs increased with respect to time while the optimum ratio of plant extract to AgNO3 solution was 1:1. The hydroxyl group was strongly involved in the bioreduction of the silver salt as indicated by the FTIR spectra. The synthesized AgNPs were crystalline in nature, with a uniformly distributed network of web-like structure. The factorial model predicted the nanoparticles yields with minimal errors. The nanoparticles were active against all the tested pathogens and thus have great potentials as anti-mibrobial agents.
Radu Socoteanu
Institute of the Romanian Academy, Romania
Title: Eco-friendly features in long-chain studies for pharmaceutical tetrapyrrolic formulation targeting efficient drug delivery
Biography:
Radu Socoteanu – senior researcher in the Molecular Structure Department of the Physical Chemistry Institute of the Romanian Academy, Bucharest - is specialized in medicinal chemistry (Ph.D. thesis “Structure, properties and application of some porphyrinic compounds”), in the field of tetrapyrrolic type compounds unconventional synthesis, related analytical and physical chemistry - various techniques from optical spectroscopy to NMR. He has over 35 ISI articles, 8 patents, 2 book chapters; director, partner co-director or member in 22 national and international projects (NATO, MNT-Era-Net, MEC-MCT, CNCSIS).
Abstract:
Porphyrinic compounds represent the main class of tetrapyrrolic structures which play an important role in photodiagnosis and photodynamic therapy of malignant tumors. Therefore, the synthesis, investigation spectral properties and in vitro evaluation at the cellular level of such structures are of current interest in medicinal chemistry. Despite the complete absence of toxicity related to the porphyrinic compounds themselves, almost all stages involving classical synthesis and most of the analytical evaluation stages involve high toxicity. Unconventional microwave synthesis proved to be in this case the main factor in reducing the number of side products and toxic solvents usually used in the classical obtaining process for acethoxy and methoxy periphery A3B porphyrin type structures. Also, the evaluation by AFM microscopy of important features, such as aggregation capacity or interaction with other structures involved in pharmaceutical formulation (e.g. polyethylene glycol or several nanoparticles) prove to be an efficient eco-friendly tool for evaluation of such nano-systems.
Andreana Morandini
University Ca’ Foscari of Venice, Italy
Title: Study of the in situ formulation of Active Condensation Agents and their use for amide formation
Biography:
Abstract:
The synthesis of covalent bonds for the formation of amides and esters is of great importance to produce pharmaceuticals, peptides, polymers, etc, these reactions often require a long time and hard conditions. A wide variety of condensing reagents is known such as acyl azides, uronium salts and carbodiimides can occur these reaction in a short time and at room temperature.
In the last decade, 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride (DMTMM) has been demonstrated to be a very efficient condensing reagent and its use is rapidly increasing due to its high activity, high solubility in water and alcoholic solvents. New alternative condensing agents are of great interest for fine chemistry synthesis to reduce costs and improve yelds.
This in mind, we developed a protocol for the in situ preparation of a library of 4-(4,6-dialkoxy-1,3,5-triazin-2-yl)-4-alkyl-ammonium halides to be used as condensing agents for the formation of amide bonds. Our preliminary results show that these quaternary ammonium salts formulated in situ, further simplify the use of these reagents, reducing their cost. Moreover, in some cases the preformed quaternary ammonium salt is unstable and decomposes quickly while the in situ formulation gives an active and efficient condensing agent. Conversions of over 80% are achieved in most cases in around 15 min.
These activating agents have been efficiently employed for the synthesis of amides and the stabilization of collagen.
Sandeep V.H.S. Bhaskaruni
University of KwaZulu-Natal, South Africa
Title: V2O5/ZrO2 as an efficient reusable catalyst for the facile, green, one-pot Synthesis of novel functionalized 1,4-dihydropyridine derivatives

Biography:
Abstract:
A practical method is designated for the one-pot, multicomponent synthesis of 1,4- dihydropyridine derivatives by cyclo-condensation of aromatic aldehydes, 5,5-dimethyl-1,3-cyclohexanedione, acetoacetanilide and ammonium acetate. Using ethanol as solvent and V2O5/ZrO2 as heterogeneous catalyst, ten novel 1,4-dihydropyridines were synthesized at room temperature (Reaction time<20 min).XRD, TEM, SEM and BET analysis were used to characterize the catalyst materials. Simple work-up, green solvent, short reaction times, moderate reaction conditions and excellent yields (90–96%) are the attractive features of this novel approach. With no need of chromatographic separation, the reaction product is easily separable in pure form.

Biography:
The development of efficient methods for the synthesis of substituted anilines with absolute regiocontrol is a central theme in organic synthesis. In that regard, we were particularly interested in developing a general procedure for C-N bond formation[1-2] via the arylation of weakly acidic amines with benzoic acids. In line with the palladium/copper co-catalyzed decarboxylative coupling rationale developed in the Goossen group[3-4], we now report silver-free conditions under which primary and secondary aliphatic amines as well as diversely substituted anilines can undergo selective mono-arylation with benzoic acids in good to excellent yields (Scheme 1).
Scheme 1 Arylation of aliphatic and aromatic amines via Pd/Cu co-catalyzed oxidative decarboxylative coupling.
This poster will focus on the significant aspects of the reaction development. Decisive for an efficient C-N bond formation is the selection of an apposite oxidant/solvent system. Strikingly, many primary aliphatic amines and also a few secondary acyclic amines can undergo N-arylation following this procedure. An example of a short synthesis of a complex substituted arylamine will be presented in which the C-N bond formation via decarboxylative coupling is an early key step in the overall process to illustrate the applicability of this process. We expect that this methodology will provide new concepts upon which the development of more environmentally friendly oxidative decarboxylative couplings can build for the future.
Abstract:
Giulia Bertoli
Ruhr Universität Bochum, Germany
Title: Metal-free trifluoromethylthiolation of arenediazonium salts with Me4NSCF3

Biography:
Abstract:
Fluorine-containing molecules are abundant in pharmaceuticals, agrochemicals and material sciences.[1] In this context, lots of attention has been given to the SCF3 group, which induces higher lipophilicity and membrane permeability to functionalised molecules.[2] Despite their importance and applicability, the synthesis of these molecules through traditional pathways is usually based on waste-intensive and multistep syntheses. The Sandmeyer reaction is one of the few processes using substoichiometric amounts of copper to achieve high yields.[3] However, for pharmaceutical applications, it is desirable to avoid the use of heavy metals. We developed a greener approach for the synthesis of trifluoromethyl thioethers starting from arenediazonium salts in which no heavy metal mediators are needed. Moreover, the reaction is completed within short times and mild conditions. Many substrates are tolerated achieving moderate to good yields. The mechanism was investigated through cyclic voltammetry studies, which highlighted a SET process from the Me4NSCF3 to the arenediazonium salt. Overall, this approach demonstrates that it is possible to perform trifluoromethylthiolation of arene electrophiles in the absence of heavy metal mediators, showing a good applicability for processes in which even ppm quantities of metals need to be avoided.
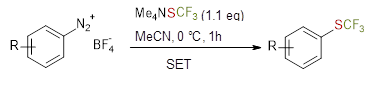
Valentina Bragoni
Ruhr Universität Bochum, Germany
Title: Synthesis of new bio-based surfactants from cashew nutshell liquid in water

Biography:
Abstract:
Cashew nutshell liquid (CNSL) is an inedible waste product (1.000.000t/a) in the cashew nut processing and is an excellent candidate for the synthesis of bio-based synthetically valuable compounds, as its production does not compete with the land use for food production.[1,2] CNSL is a mixture of phenols bearing a 15-carbon side chain with different degrees of unsaturation.[3,4] We have developed an eco-friendly and waste minimised concept for the synthesis of amine-based surfactants from CNSL. The key step of the procedure is a reductive amination of CNSL with molecular hydrogen in water as solvent, and palladium on charcoal as catalyst. The resulting cyclohexyl amines are successfully converted into N-oxide, betaine and quaternary ammonium tensides. Their surfactant properties (surface tension and critical micellar concentration) have been determined and resulted comparable with those of state-of the-art commercial tensides, opening up a wealth of commercial applications. In the case of the particularly valuable N-oxide surfactant, a one pot synthesis with a remarkably low E-factor of 2 was realised in water as the sole solvent, with a hydrogen peroxide oxidation and purification by simple water extraction, thus avoiding the use of waste-intensive purification techniques. Overall, the process provides a new, eco-friendly procedure for the transformation of renewable waste products into industrially valuable compounds.
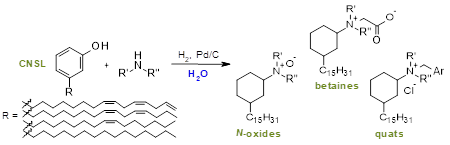
Guodong Zhang
Ruhr-Universität Bochum, Germany
Title: Regioselective C–H Alkylation via Carboxylate-Directed Hydroarylation in Water

Biography:
Abstract:
In the presence of catalytic [Ru(p-cym)Cl2]2 and using Li3PO4 as the base, benzoic acids react with olefins in water to afford the corresponding 2-alkylbenzoic acids in moderate to excellent yields. This C–H alkylation process is generally applicable to diversely substituted electron-rich and electron-deficient benzoic acids, along with a,b-unsaturated olefins including unprotected acrylic acid. The widely available carboxylate directing group can be removed tracelessly or utilized for further derivatization. Mechanistic investigations revealed that the transformation proceeds via a ruthenacycle intermediate.
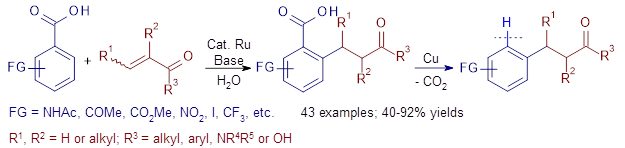
Zhiyong Hu
Ruhr-Universität Bochum, Germany
Title: Carboxylate-Directed C–H Allylation with Allyl Alcohols or Ethers

Biography:
Abstract:
A [Ru(p-cymene)Cl2]2 catalyst activates allyl alcohols and ethers for the regioselective ortho-C–H allylation of aromatic and heteroaromatic carboxylates. The reaction is orthogonal to most C–H functionalizations with allyl alcohols in that allyl arenes rather than carbonyl compounds are obtained. A wide range of substrates are thus smoothly transformed to allylarenes at 50 °C in phosphate-buffered 2,2,2-trichloroethanol. The reaction concept combines the use of abundant reagents and directing groups in a sustainable, waste-minimized method for C–C bond formation.
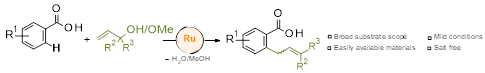
Amara Dar
University of the Punjab
Title: Study of the removal mechanism of chromate ion from water; the potential human carcinogen

Biography:
Dr.Amara Dar has completed her PhD in Feb.2018from Institute of Chemistry, University of the Punjab Lahore. Earlier she has done MS (Analytical Chemistry) and BSc.(hons) Chemistry from the same Institution. She has served Center for Undergraduate Studies, University of the Punjab as Lecturer from 2007 till Feb.2018 and at present working as assistant professor. She has published 19 reserach papers in well reputed impact factor journals. She is author of a monograph and a book.
Abstract:
Chromium is potential human carcinogen is major component of various industrial effluents. Removal of chromium using various methods has been reported in the literature. Using adsorption technique chromium in the form of chromate was removed in the present study. In countries like Pakistan resources are limited and a cost effective and environmentally benign method is needed to facilitate the community. In order to reduce the effect of chromium pollution novel adsorbents with remarkable adsorption capacities were practiced during this study. Acid treated arjun nuts, beerri ptta capsule and multani mitti along with untreated amberlite IRA 410 were employed for the batch-wise removal of chromate ions from water. In depth mechanism was explored by applying various isothermal model and adsorption capacity (mg/g) of amberlite and acid treated arjun nuts revealed their remarkable potential for chromate adsorption. Time dependence and temperature effect was also studied by applying kinetics and thermodynamics model.
Malika MOKHTARI
University of Tlemcen, Algeria
Title: Photodegradation of 2,4,6-Trichlorophenol using naturel hematite modified with Chloride of Zirconium Oxide
Biography:
Malika Mokhtari has completed her Doctorate from Andhra University and postdoctoral studies from University of Tlemcen in Algeria. She is the head a research laboratory since 2009. She has published more than 15 papers in reputed journals and has been serving as revewer.
Abstract:
The catalyst NHZr was synthesized using natural hematite and chloride of zirconium oxide. NHZr was characterized by FT-IR, BET and DRX. This catalyst was applied to photocatalyse 2,4,6-trichlophenol under UV254 nm irradiation. Effect of different parameters such as pH, catalyst concentration, pollutant concentration were studied. The results showed that 96% of 2,4,6-trichlorophenol was degraded in 180 min of treatment with optimum operating conditions of 0.5 g/L of photocatalyst, pH medium and 10 mg/L 2,4,6-trichlorophenol. COD elimination and hydrogen peroxide formation were also investigated. Kinetic results showed that photocatalytic reaction obeyed pseudo-first-order .
The NHZr was manufactured by products available, easy to handle, effective on recalcitrant products and has a recyclable character, which gives great value to this photocatalyst.
Lili Zhen
University of Roma Tor Vergata, Italy
Title: A holistic approach to tannins chemical functionalization towards tailored surface adsorpiton properties
Biography:
Lili Zhen obtained her Master's degree in Textile Chemistry, Dyeing & Finishing Engineering from the National Key Laboratory of Eco-Textile of the Jiangnan University in China in 2016, with an experimental thesis entitled ‘Thermoplastic modification research of rice straw through graft copolymerization with PEG’. Prior to this, in 2013, Lili Zhen obtained her Bachelor's degree from Jiangnan University working on Light Chemistry Engineering. In 2016, Lili Zhen was chosen as a PhD student within the H2020 program BIOCLEAN under the supervision of Prof. C. Crestini at the University of Roma 'Tor Vergata', Italy.
Abstract:
Tannins are a major source of polyphenolic components after lignin, with 160 000 tons potentially biosynthesized each year. Due to their excellent protein complexing abilitity,tannins have different metabolic and biological roles, such as cell wall construction, against worms, fungi, bacterials and UV protection, that made tannins perfect natrual biofilm control agents in human daliylife life. Tannins posess multiple structure units featured on aromatic oligmer or macromolecular with free phenolic groups and usually classified into hydrolysable tannins that consist of esters of gallic acid with a core sugar, and condensed tannins that are oligomers or polymers of flavan-3-ol units.
The aim of our work has focused on the tayloring of hydrophobic/hydrophilic properties of different tannins. A general chemical process for specific tannin functionalization has been developed and a library of specifically functionalized tannins carrying additional functional groups in specific loading factors has been created. More specifically ammonium groups, carboxylic groups and PEG have been succesfully linked to an array of five different tannins possessing different chemical structures.The products were fully characterized by quatitative 31P NMR, 1H NMR, HSQC, MALDI.
CHEN Fengming
Chinese Academy of Inspection and Quarantine, China
Title: Fragmentation pathway of harmful chemicals in soft ionization mode and its application in novel analogue screening
Biography:
Abstract:
The MS/MS fragmentation pathway of the cinnamic esters additives was illustrated. There were four analytes (butyl heptanoate, ethyl cinnamate, n-propyl cinnamate and benzyl cinnamate) had m/z 131 with higher abundance. By analyzing the structure of compound ethyl cinnamate, n-propyl cinnamate and benzyl cinnamate, these similar fragments were due to consecutive losses of the cinnamoyl moiety (m/z 131). The mechanisms show the common fragmentation behavior of the cinnamic esters.The fragmentation mechanism of mass spectra of these β-agonists show that dehydration reactions occurred in the pathways of each compound. A common characteristic was observed that there was neutral loss of a water molecule for all β-agonists. And for 7 β-agonists, loss of an alkyl group from the secondary amine was demonstrated. Moreover, m/z at 74 and 60 were for the butyl and propyl derivatives respectively.
The fragmentation pathway of crocin, crocetin and geniposide were studied. The DP and CE values of crocin were higher than those of crocetin which may be because of the stable gentiobiosyl bond and large molecular weight of crocin. The precursor ion [M-H]− (m/z 975) of crocin had potential to loss gentiobiosyl (m/z 324) for [M-H-gen]− (m/z 651) and to loss 2 gentiobiosyl (m/z 648) for crocetin (m/z 327).
Seong Ju Kim
Hankyong National University, South Korea
Title: Bio cruode oil production as combustion fuels through hydrothermal liquefaction under homogeneous alkali catalyst from Korean native kenaf
Biography:
Abstract:
Hydrothermal liquefaction (HTL) is a promising technology for producing high density liquid fuels from kenaf, a herbaceous biomass. Also, high density liquid fuels through HTL can be used to conbustion fuels as heavy oil in power plant. HTL was used to process Korean native kenaf as energy crops to produce bio crude oil, bio char, gas and aquous phase products. In this study, in order to determine the optimal condition of HTL process, the kenaf was treated using 1-7% (w/w) NaOH at 250-350 °C for 120 min. Subsequently, the mixed products (bio crude oil, bio char, and aqueous phase) were extracted by dichloromethan to only obtain bio crude oil. As a results, bio crude oil yield was approximately 20 % and bio char was produced approximately 10%. And, gas phase was mainly composed carbon dioxide and methane. After HTL process, kenaf convert to liquid fuels as conbustion fuels with high density, high calroie value, and high carbon content. The HTL process are effective procedures to produce the high quality bio crude oil.
Ga Hee Kim
Hankyong National University, South Korea
Title: Extraction and characterization of lignin from herbaceous biomass of Korea via organosolv and alkaline process
Biography:
Abstract:
The aim of the present study was to investigate the effect of extraction conditions on the molecular structure of alkaline and organosolv lignins extracted from Korea native herbaceous(Miscanthus and Kenaf). The lignin fractions obtained were then characterized by EA, ICP-AES, TGA, GPC, FT-IR, NMR, Py-GC/MS, and sugar analysis. The structural characterization of lignin will be improving the understanding of complex lignocellulosic biomass pretreatment which is vital for the production of biofuels and phenolic compounds. Comparing the two pretreatment methods, it was found that Mn, Mw and polydispersity of the lignin extracted with ethanol solutions was lower than lignin extracted with alkaline solutions. Elemental composition of Lignins increased in carbon content, and the oxygen content, as expected, decreased. FTIR analysis of pretreated solid residues revealed reduction in p-hydroxyphenyl (H), guaiacyl (G) and syringyl (S) lignin, FTIR and p-NMR of these lignins showed S, H and G units. The lignin’s physical and chemical behavior was seen different with respect to the extraction method used.
Kasitnun Chayavanich
Chulalongkorn University, Thailand
Title: Biocompatible film based on corn starch/gelatin and red radish anthocyanin for pH sensing

Biography:
Abstract:
The pH-sensitive films were developed using starch/gelatin loaded with red radish anthocyanin for meat spoilage observation. This work combined several benefits which can possibly utilize in food samples, including bio-compatible material and natural dye based, low cost, fast response, and ease of use. The colors of films could be differentiated by naked eye changing from orange to grey-purple at pH 2-12 and captured by smartphone in the studio light box. The color parameters were evaluated by image J software. To confirm that red radish anthocyanin could incoporate to polymers, films were characterized by FTIR, SEM and AFM. Regarding the color stability trial, the results showed the preferable storage temperature of films was refrigeration temperature. Furthermore, the pH-sensitive films were applied to food samples for real-time meat spoilage observation, the results suggested that films could be used as intelligent food packaging.
- Biomass and its Conversion | Environmental Chemistry and Pollu on Control | Green Chemistry and Technology
Location: Meeting Place 2

Chair
Bharthi Khungar
Birla Institute of Technology and Science (BITS) Pilani, India
Session Introduction
Rabya Aslam
University of the Punjab, Pakistan
Title: Alumina Extraction from Pakistani Bauxite and activity testing on biofuel production from waste cooking oil
Time : 10:00-10:30

Biography:
Rabya Aslam has completed her PhD from University of Erlangen Nuremberg Germany in 2016. Currently, she is working as Assistant Professor at University of the Punjab, lahore Pakistan. She has published more than 15 papers in repured journals.
Abstract:
Waste cooking oil is valuable and cheap feedstock for the production of bio-fuels as compared to virgin edible oil. It can not only help to reduce environmental impacts of waste cooking oil but also can contribute to the future energy demand. Catalysts used in this process are usually acids, base in both homogeneous and heterogeneous processes. In most cases, sodium hydroxide and potassium hydroxide are used as alkaline catalyst and mineral acids are used as acidic catalysts in homogeneous reaction, because of their higher reaction rates, availability and low cost. However, recovery of catalyst is difficult in this process. Moreover, in the case of waste cooking oil which contain relatively high percentage of free fatty acid, alkaline catalysts are prone to the saponification reaction which reduce the biodiesel conversions. In order to cope up with low biofuel conversion, slow reaction rates, activity of solid catalysts are evaluated in this work. The activity of in house synthesized Alumina from Pakistani Bauxite is studied by varying reaction conditions such as time, temperature, alcohol to oil ratio. The produced biodiesel was fully characterized with respect to density, kinematic density, iodine values, acid values, carbon residue, pour points, flash points etc. and was found to meet the criteria required to be diesel substitute.
Fionn Ó Fearghail
Dublin Institute of Technology, Ireland
Title: Optimised solid state 13C CP-MAS NMR, FTIR and Raman spectroscopy for the accurate determination of %Degree of Acetylation of extracted crab shell chitin for valorisation of fisheries waste streams
Time : 10:50-11:20

Biography:
Fionn Ó Fearghail is a postgraduate researcher in the Radiation and Environmental Science Centre (RESC) and NanoLab in the FOCAS Institute at the Dublin Institute of Technology. His research focuses on development of analytical techniques for the detailed characterization of marine sourced bio-polymers and bio-active compounds. Fionn also works towards up-scaling of sustainable processing and extraction of raw materials for valorisation of waste streams by enhancing understanding of the chemical and physical properties of raw and extracted marine sourced materials.
Abstract:
Solid state 13C CP-MAS NMR was optimised for the accurate determination of %Degree of Acetylation (%DA) using a range of extracted chitin and chitosan standards. Application of the optimised 13C CP-MAS NMR technique, along with FTIR and Raman spectroscopy for qualitative analysis, determined the %DA of fisheries waste-stream chitin isolated from brown crab, Cancer Pagurus, as being from 91-100% and of high purity. The extraction procedure was optimised, under principles of green chemistry and for ease of up-scaling for industrial application, by review of literature and replicate studies. Extraction is shown to be necessary for accurate analysis as determined by comparison of 13C CP-MAS NMR, FTIR and Raman spectra of both raw and extracted samples. An analytical suite consisting of these techniques is proposed as a standard reproducible method for the accurate characterisation of crustacean sourced chitin.
Axel Kosider
Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Germany
Title: Recycling of heterogeneous catalysts for the room-temperature decomposition of aqueous formic acid mixtures
Time : 11:20-11:50

Biography:
Axel Kosider graduated with a Master’s Degree in Chemical Engineering from the Technical Faculty at the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). Kosider became interested in research at the Lehrstuhl für Chemische Reaktionstechnik (CRT) during his semester abroad in South Korea at the FAU Busan Campus. During his studies, Kosider worked as a research assistant at CRT, where he then wrote his master’s thesis. In 2015 he began his PhD in the research group of Prof. Dr. Peter Wasserscheid.
Abstract:
Alternatives to fossil fuels as an energy source are necessary to reach a sustainable tomorrow. A green, renewable and promising energy carrier is formic acid that can be decomposed to hydrogen and carbon dioxide. With a hydrogen capacity of 4.3 wt%, formic acid delivers hydrogen that can be converted to electricity in fuel cells. The decomposition of the acid takes place at mild reaction conditions. With heterogeneous palladium catalysts, the dehydrogenation of aqueous formic acid happens at room temperature and ambient pressure with high selectivity of hydrogen and carbon dioxide. Because of the moderate reaction conditions, hydrogen is obtained on-demand and can be converted to energy. The source for sustainable, green and renewable formic acid is the conversion of biomass to aqueous formic acid. With the help of homogeneous catalysts, biological waste is converted to aqueous formic acid that can be used as green energy carrier. However, while decomposing aqueous formic acid to hydrogen and carbon dioxide, heterogeneous palladium catalysts deactivate within a short time scale. Within a few hours, the activity of the catalyst decreases dramatically so that it is necessary to regenerate the catalytic active material. Regenerating the poisoned heterogeneous palladium catalysts is possible so that the precious metal can be reused for the dehydrogenation of aqueous formic acid. By recycling the palladium catalysts, it is feasible to use formic acid as a renewable and green liquid hydrogen storage chemical.
Dina A. Ahmed
Future university in Egypt, Egypt
Title: Double-Dip Approach: Simultaneous Acquisition of the Dissolution Curves of Two Active Ingredients in a Binary Pharmaceutical Dosage form Exploiting the Opportunities Offered by Ion Selective Electrodes
Time : 11:50-12:20
Biography:
Mrs. Dina Abbas Ahmed Mostafa has completed her Master's degree in Pharmaceutical Sciences on September 2015 from Ain Shams University and has been enrolled on May 2016 for the Ph.D. degree as a Ph.D. student and still registered for this degree. She is currently working as assistant lecturer at faculty of pharmaceutical sciences and pharmaceutical industries at Future University in Egypt, Cairo, Egypt.
Mrs. Dina Abbas participated in many local and international conferences:
- 3rd FUE International Conference of Pharmaceutical Sciences ( February 9-11, 2015, Cairo, Egypt)
- 2nd Annual International Conference on Pharmaceutical Sciences ( May 4-7, 2015, Athens, Greece)
- 4th FUE International Conference of Pharmaceutical Sciences ( 31 January-2 February , 2017, Cairo, Egypt)
- 8th International Scientific Conference ( March 2-3, 2017, Cairo, Egypt)
Abstract:
Acquisition of the dissolution profiles of more than single active ingredient in a multi-component pharmaceutical formulation is dominated by utilization of the off-line spectroscopic and chromatographic methods. In this approach, a “Double-Dip” green analytical chemistry (GAC) approach with the ultimate goal of advancing the in-line potentiometric sensors to their most effective use for simultaneous acquisition of the dissolution curves of two active ingredients in a binary pharmaceutical dosage form, Brufen Flu is adopted. For the proof of concept, two sensitive and selective sensors were developed for the simultaneous determination of the cationic Pseudoephedrine (PSE) and the anionic Ibuprofen (IBU) drugs in order to monitor their dissolution profiles without sample pretreatment or derivatization. For the determination of the cationic drug (PSE), sensor I was developed using potassium tetrakis (4-chlorophenyl)borate (KTCPB) as a cationic exchanger, while sensor II was developed for the determination of the anionic IBU using tridodecyl methyl ammonium chloride (TDC) as an anionic exchanger using 2-nitrophenyl octyl ether (2-NPOE) as a plasticizer for both used sensors. The use of these novel sensors not only provides a way for the determination of PSE and IBU in bulk powder, in laboratory mixtures and in combined dosage form but also permits simultaneous in-line monitoring of their dissolution profiles. The advantages of the newly introduced “Double-Dip” approach are highlighted and the merits of these benign real-time analyzers (ISEs) that can deliver equivalent analytical results as HPLC and UV-spectrophotometry while significantly reducing solvent consumption/waste generation in addition to the manipulation steps are described.
Nadia El Ouahedy
University of Poitiers, France
Title: Preparation of activated carbon from olive waste. Application as adsorbent for persistent organic pollutants in water
Time : 12:20-12:50

Biography:
Abstract:
In the present study, we have investigated the adsorption, by activated carbon prepared from olive stones, of two pollutants, Bisphenol A, a substance causes a disruption of endocrine systems and ubiquity in the aquatic environment, and Diuron, a pesticide detected in groundwater and may reach higher levels than health-based standards. The olive stones were chemically activated and then pyrolysed (thermal treatment under nitrogen). On the other hand, to optimize the preparation method, the effect of the main process parameters (such as activating agent used, impregnation ratio, temperature of pyrolysis step) on the performances of the obtained activated carbons (expressed in terms of adsorption capacity of BPA and Diuron and specific surface area) was studied. The physicochemical properties of the activated carbon prepared were characterized by N2 adsorption/desorption, FTIR, SEM, X-Ray diffraction, CHNS and TGA/DTA. To optimize the adsorption parameters of the activated carbon, preliminary experiments were achieved, such as the effects of solution initial pH and temperature, effect of initial concentration of the pollutants. Promising performances were pointed out as 70 % of Diuron and 92 % of BPA can be removed from aqueous solution for an initial concentration respectively 35 mg/L and 20 mg/L, when the usual concentrations of BPA in environmental waters are in the range of 10 ng/L to 400 μg/L and Diuron is around 1 600 ng/L. This innovative process is based on valorization of agricultural waste biomass, of which billions of kilograms are produced annually, to low cost but efficient adsorbent that can contribute to environmental remediation.
Jialu Li
Université de Poitiers, France
Title: Catalytic transformation of 2,5-Dimetylfuran to functionalized 2-methyltetrahydrofuran derivatives
Time : 12:50-13:20

Biography:
Jialu Li is currently pursuing her PhD at University of Poitiers.
Abstract:
Furans as well as its derivatives are platform chemicals, subsequently transformed to value-added products such as pharmaceutical intermediates, fuel additives, bio-based surfactant and solvents. 2,5-dimetylfuran (DMF), a prominent platform molecule, can be obtained via the selective hydroghenation of 5-hydroxymethylfurfural [1]. Despite its outstanding properties, nearly ideal boiling point, high energy density, high research octane number and being immiscible with water[2], it can also be further valorised. Nonetheless, the amount of furanic derivatives synthesized from DMF is limited due to the complexity of the functionalization of the methyl group. Here, for the first time, we described a convenient catalytic pathway to functionalize methyl group of DMF by using hydrolyzation product 2,5-hexanedione[3]as key an intermediate.
A three-step strategy is employed (Figure 1). Starting from (1) 2,5-dimethylfuran, hydrolyzation of DMF to hexane-2,5-dione is performed in presence of an acid catalyst; (2) then hexane-2,5-dione reacts with and aldehyde in the presence of a basic catalyst; followed by (3) a hydrogenation/cyclization by metal support catalyst to obtain methyltetrahydrofuran derivatives.
Figure 1: Strategy to synthesis functionalized 2-methyl THF derivatives
The screening of acid catalysts for the first step and basic-catalysts for the second step was investigated leading to interesting yields to the desired products. Base on these results, the feasibility of one-pot reaction starting directly from 2,5-DMF and aldehydes was demonstrated. The third step (hydrogenation/cyclization) was studied and 80% of the final target product was achieved. A theoretical approach combined with an experimental approach revealed that the reaction temperature is a key parameter in this step. The recyclability of the catalysts in all the three steps was also studied.
Kevin Speina
Princeton University, USA
Title: Towards commodity scale production of ketones using a resilient fungal heme protein
Time : 13:20-13:50
Biography:
Kevin Speina is a graduate student in the Department of Chemistry at Princeton University. Currently, he is pursuing his PhD in Bioinorganic Chemistry. He has graduated from Manhattan College with a BS in Chemistry and Minors in Philosophy, Religion, and Mathematics. His interests in green chemistry and entrepreneurship has kindled a passion for pursing research and development; particularly focused on bringing products from lab bench to market. He is a first-generation student for both his Bachelor’s and Doctoral degree.
Abstract:
The non-reactive nature of aliphatic C-H bonds makes catalytic oxyfunctionalization, under ambient conditions profoundly difficult. Overcoming this challenge has the potential of transforming low cost, abundant hydrocarbons (i.e. methane, cyclooctane) into low cost precursor molecules capable of derivatization; an industrially advantageous enterprise. Fortunately, nature evolved enzymatic catalysts able to perform oxygenation onto relatively inert C-H bonds. Cytochrome P450s (P450) are a class of monooxygenases that incorporate molecular oxygen onto aliphatic C-H bonds with remarkable regio- and stereoselectivity. Unfortunately, P450 enzymes require a prohibitively expensive cofactor NAD(P)H and reductases to perform oxyfunctionalization reactions. A class of enzymes known as fungal aromatic peroxygenases (APOs) circumvents this limitation by using H2O2 as a co-substrate to generate the iron (IV) oxo porphyrin cation radical intermediate for C-H functionalization. APO enzymes are stable, extracellular, glycosylated proteins that have no sequence homology to P450 analogs yet have enhanced reactivity to P450 enzymes. Herein, a recently identified APO from Marasmius rotula [MroAPO] was found to be stable in the presence of organic solvents such as acetonitrile and acetone (up to 50v%). Even more remarkable is its propensity to form ketones from alkanes in high yields. Simply by adding 0.05% mol of MroAPO in acetone with excess oxidant, alkane substrates such as cyclooctane and decane are transformed to the corresponding ketone products with 90+% yield. A total turnover number (TTN) of 50000 was calculated for cyclooctane oxidation. This is the second highest TTN reported for cyclooctane. Astonishingly, enzymatic reactions with C10-C6 linear alkanes results in the 2-ketone product, with minute amounts of side products. The 2-ketone products are desirable for the fragrance and food industries. Thus, the reactions catalyzed by MroAPO enzymes have promising biotechnical potential insofar their versatility is unmatched to any known catalyst.