
Dominik Lichte
Ruhr Universität Bochum, Germany
Biography
The development of efficient methods for the synthesis of substituted anilines with absolute regiocontrol is a central theme in organic synthesis. In that regard, we were particularly interested in developing a general procedure for C-N bond formation[1-2] via the arylation of weakly acidic amines with benzoic acids. In line with the palladium/copper co-catalyzed decarboxylative coupling rationale developed in the Goossen group[3-4], we now report silver-free conditions under which primary and secondary aliphatic amines as well as diversely substituted anilines can undergo selective mono-arylation with benzoic acids in good to excellent yields (Scheme 1).
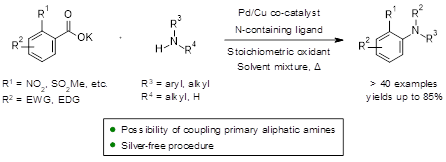
Scheme 1 Arylation of aliphatic and aromatic amines via Pd/Cu co-catalyzed oxidative decarboxylative coupling.
This poster will focus on the significant aspects of the reaction development. Decisive for an efficient C-N bond formation is the selection of an apposite oxidant/solvent system. Strikingly, many primary aliphatic amines and also a few secondary acyclic amines can undergo N-arylation following this procedure. An example of a short synthesis of a complex substituted arylamine will be presented in which the C-N bond formation via decarboxylative coupling is an early key step in the overall process to illustrate the applicability of this process. We expect that this methodology will provide new concepts upon which the development of more environmentally friendly oxidative decarboxylative couplings can build for the future.
Abstract
Abstract : Decarboxylative Amination Of Benzoic Acids

