
Jialu Li
Université de Poitiers, France
Title: Catalytic transformation of 2,5-Dimetylfuran to functionalized 2-methyltetrahydrofuran derivatives
Biography
Biography: Jialu Li
Abstract
Furans as well as its derivatives are platform chemicals, subsequently transformed to value-added products such as pharmaceutical intermediates, fuel additives, bio-based surfactant and solvents. 2,5-dimetylfuran (DMF), a prominent platform molecule, can be obtained via the selective hydroghenation of 5-hydroxymethylfurfural [1]. Despite its outstanding properties, nearly ideal boiling point, high energy density, high research octane number and being immiscible with water[2], it can also be further valorised. Nonetheless, the amount of furanic derivatives synthesized from DMF is limited due to the complexity of the functionalization of the methyl group. Here, for the first time, we described a convenient catalytic pathway to functionalize methyl group of DMF by using hydrolyzation product 2,5-hexanedione[3]as key an intermediate.
A three-step strategy is employed (Figure 1). Starting from (1) 2,5-dimethylfuran, hydrolyzation of DMF to hexane-2,5-dione is performed in presence of an acid catalyst; (2) then hexane-2,5-dione reacts with and aldehyde in the presence of a basic catalyst; followed by (3) a hydrogenation/cyclization by metal support catalyst to obtain methyltetrahydrofuran derivatives.
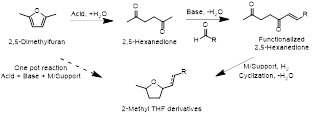
Figure 1: Strategy to synthesis functionalized 2-methyl THF derivatives
The screening of acid catalysts for the first step and basic-catalysts for the second step was investigated leading to interesting yields to the desired products. Base on these results, the feasibility of one-pot reaction starting directly from 2,5-DMF and aldehydes was demonstrated. The third step (hydrogenation/cyclization) was studied and 80% of the final target product was achieved. A theoretical approach combined with an experimental approach revealed that the reaction temperature is a key parameter in this step. The recyclability of the catalysts in all the three steps was also studied.

