Giulia Bertoli
Ruhr Universität Bochum, Germany
Title: Metal-free trifluoromethylthiolation of arenediazonium salts with Me4NSCF3
Biography
Biography: Giulia Bertoli
Abstract
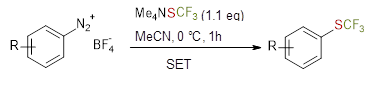
Fluorine-containing molecules are abundant in pharmaceuticals, agrochemicals and material sciences.[1] In this context, lots of attention has been given to the SCF3 group, which induces higher lipophilicity and membrane permeability to functionalised molecules.[2] Despite their importance and applicability, the synthesis of these molecules through traditional pathways is usually based on waste-intensive and multistep syntheses. The Sandmeyer reaction is one of the few processes using substoichiometric amounts of copper to achieve high yields.[3] However, for pharmaceutical applications, it is desirable to avoid the use of heavy metals. We developed a greener approach for the synthesis of trifluoromethyl thioethers starting from arenediazonium salts in which no heavy metal mediators are needed. Moreover, the reaction is completed within short times and mild conditions. Many substrates are tolerated achieving moderate to good yields. The mechanism was investigated through cyclic voltammetry studies, which highlighted a SET process from the Me4NSCF3 to the arenediazonium salt. Overall, this approach demonstrates that it is possible to perform trifluoromethylthiolation of arene electrophiles in the absence of heavy metal mediators, showing a good applicability for processes in which even ppm quantities of metals need to be avoided.